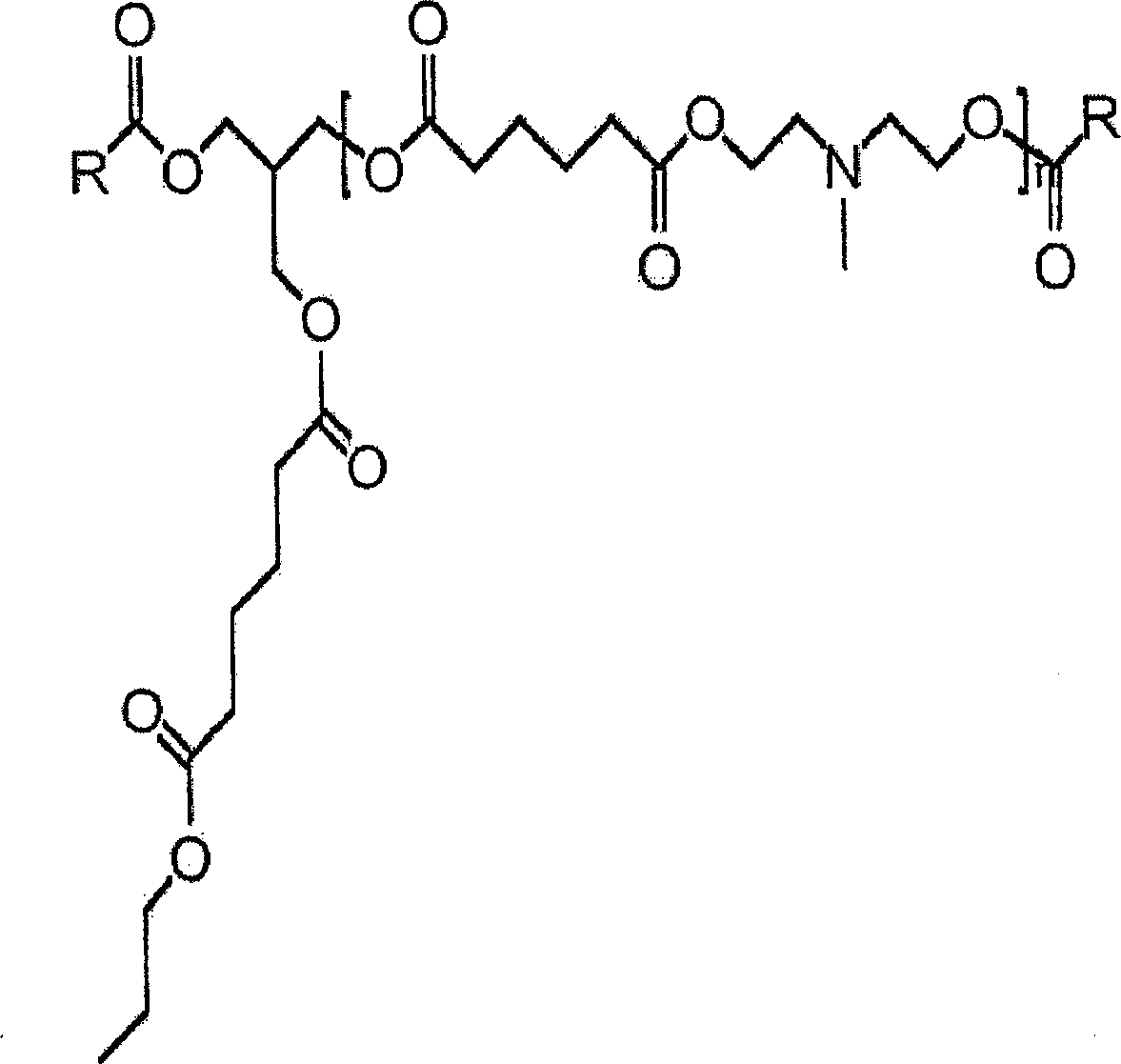
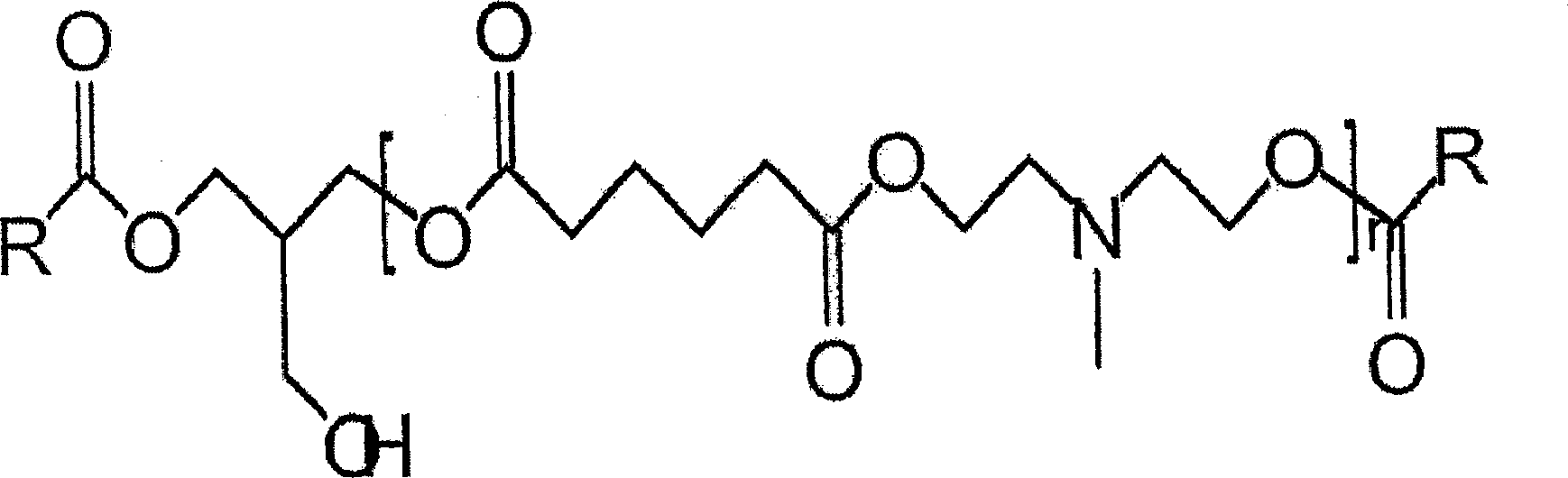
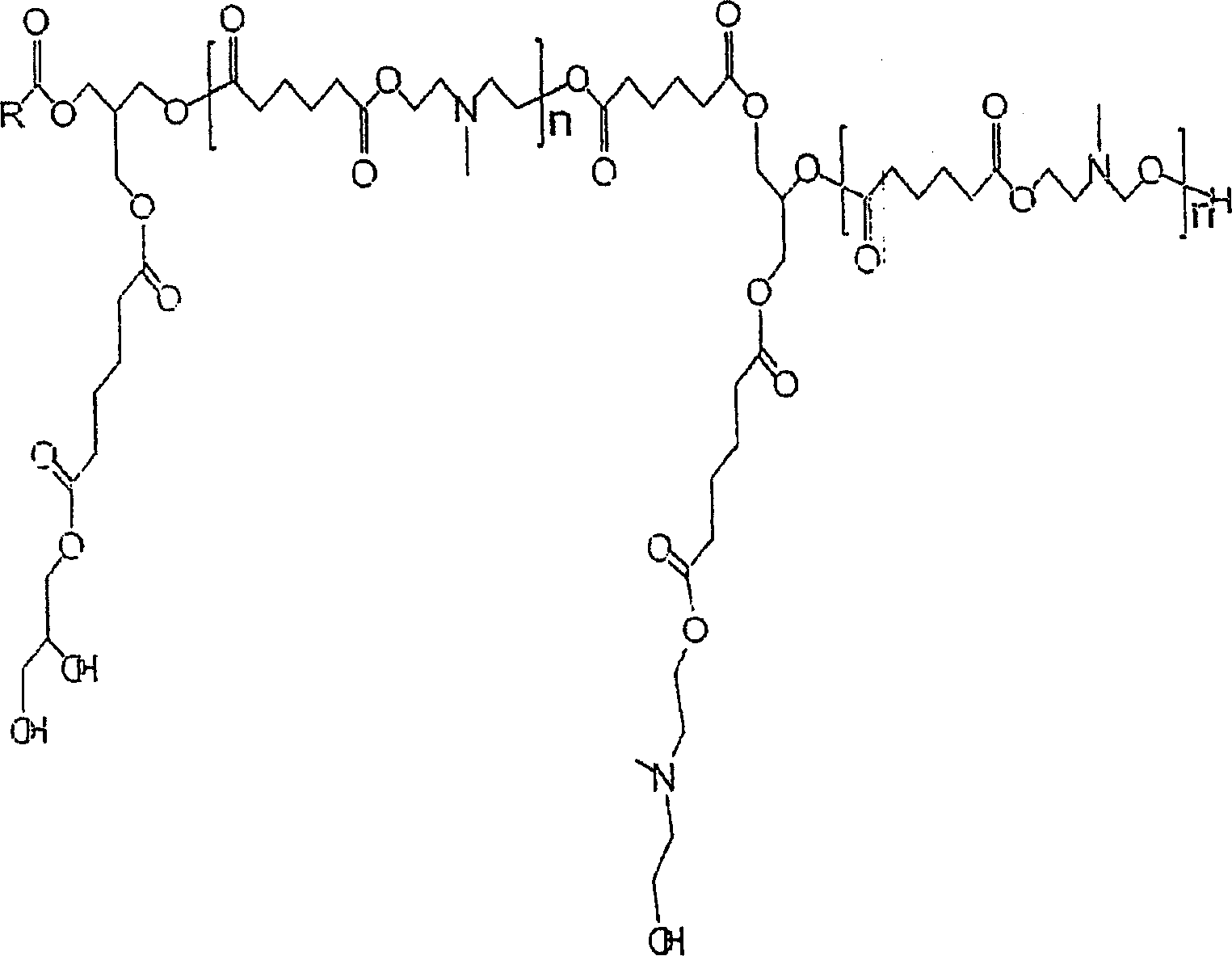
Tertiary amine functional complex polyester polymers and methods of production and use
A technology of tertiary amine groups and monofunctional groups, which is applied in the field of composite polyester polymers with tertiary amine functional groups and its preparation and application, can solve the problems of lack of cleanliness and poor emulsification performance, and achieve good lubricating performance and good bioavailability Effects of degradability, strong skin and hair compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] To evaluate the present invention, an initial matrix of prototypes was grown to evaluate its performance. Each test polyesteramine sample is designated as Type 1-6 respectively. Table 1 lists the number of moles of each component in each sample. By varying properties such as molecular weight, alkyl chain type and density, polyol type and density, and tertiary amine group density, the structure and properties of each sample can be controlled.
[0033] The samples were all prepared by feeding the components into a stirred batch reactor with a small amount of antioxidant to preserve the color. The reactants are heated to about 170°C to 200°C under a continuous sparge of inert gas. The acid and ammonia values were monitored, and when the acid value reached 10 or less, the reaction was stopped by cooling.
[0034] components
Embodiment 2
[0036] The affinity of polyesteramine samples was tested in-house using the Rubine Dye test method. In the magenta dye test, a commercial esterquat, Dehyquart(R) L-80 (dicocoyl ethyl hydroxyethyl dimethyl sulfate (and) propylene glycol) was used as a reference. The materials and equipment used to perform the magenta dye analysis are as follows:
[0037] Bleach blonde hair (De Meo Brothers);
[0038] Lumicrease Bordeaux 3LR powder-dye (Clariant);
[0039] Glacial acetic acid, UPS grade;
[0040] Hydrogen peroxide, 10% solution;
[0041] glue sprayer;
[0042] glue stick;
[0043] plastic sheet;
[0044] Colorimeter (Minolta CR-300);
[0045] Digital camera (Olympus digital camera, model C2020Z);
[0046] Polyesteramine type 1;
[0047] Polyesteramine type 2;
[0048] Polyesteramine type 3;
[0049] Polyesteramine type 4;
[0050] Polyesteramine type 5;
[0051] Polyesteramine type 6; and
[0052] Dehyqyart L-80 (Cognis)
[0053] The procedure used for the magenta...
Embodiment 3
[0065] A second group of polyesteramine products was designed and synthesized to further explore structure / property relationships with molecules surrounding structural features in the better performing Type 5 and Type 6 polyesteramine products. Still adopt the aforementioned synthetic method. Table 3 lists the results obtained from the series. The above materials were evaluated for their affinity using the magenta dye test. The results are listed in Table 4.
[0066] components
[0067] test material
[0068] The results of the magenta dye test on the modified polyesteramine samples showed that virtually all polyesteramines had better substantivity than the commercial conditioning additive Lexamine(R) S-13, with samples C1450 and Type 6 having the best substantivity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com