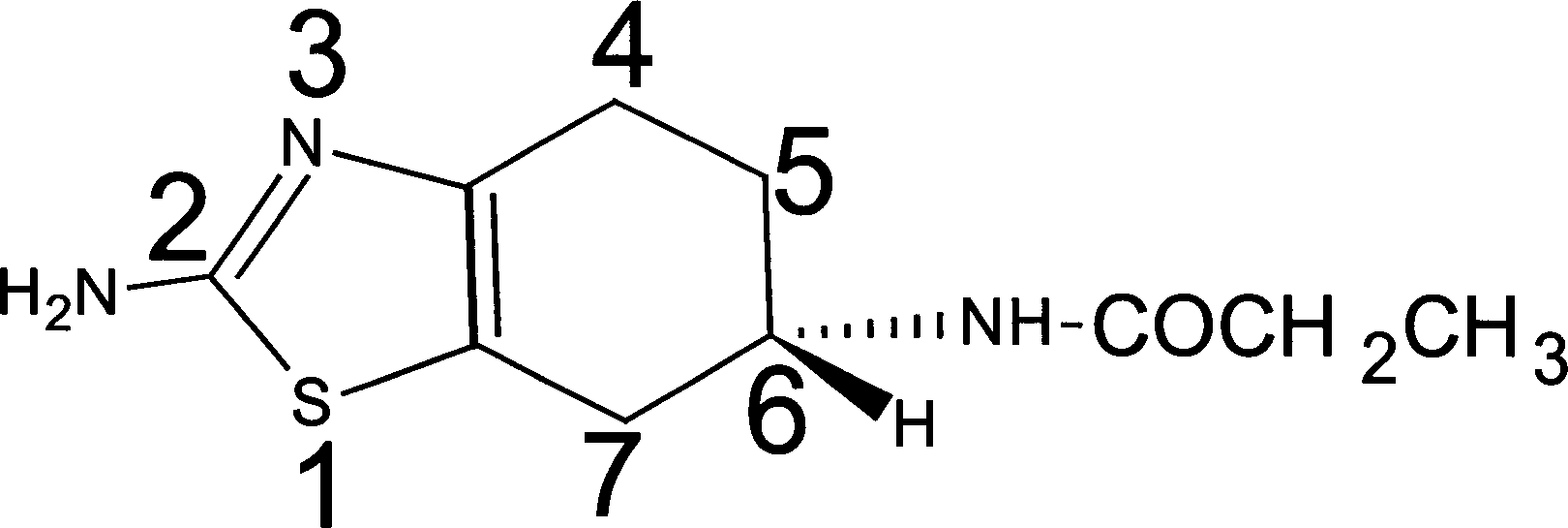
Synthesis of (-)-2-amino-6-propionamido tetrahydro benzothiazole
A technology of propionamidotetrahydro and benzothiazole, which is applied in the direction of organic chemistry, can solve the problems of high cost of raw materials, waste, and complicated operation, and achieve the effects of simplifying production process conditions, realizing industrialized production, and facilitating industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention can be realized through the following specific steps.
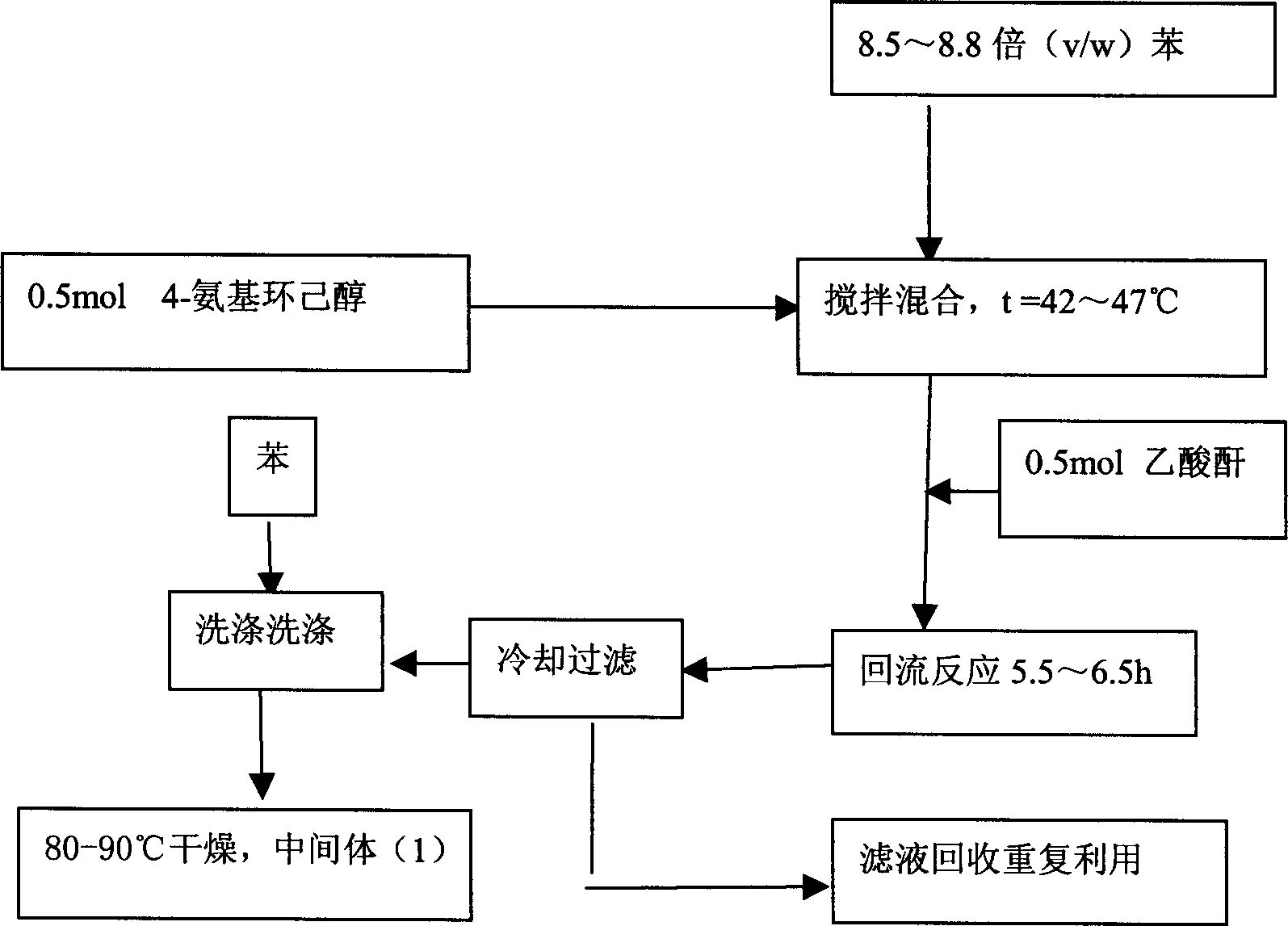
[0020] The first step reaction: Synthesis of 4-acetamido cyclohexanol (1)
[0021] Add 0.5 mol of 4-aminocyclohexanol into a dry reaction flask, then add 8.5 to 8.8 times (v / w) benzene, stir and mix, and the solution is a milky white suspension. Control the reaction temperature at 42-47°C, add 0.5 mol of acetic anhydride, after the addition of acetic anhydride, heat up to reflux for 5.5-6.5 hours, white solids adhere to the wall and bottom of the container. Stop heating, cool to below 10°C, filter, wash the filter cake with an appropriate amount of benzene, and blow dry at 80-90°C to obtain white powdery intermediate product (1), yield 95-98%, mp 158-162°C.
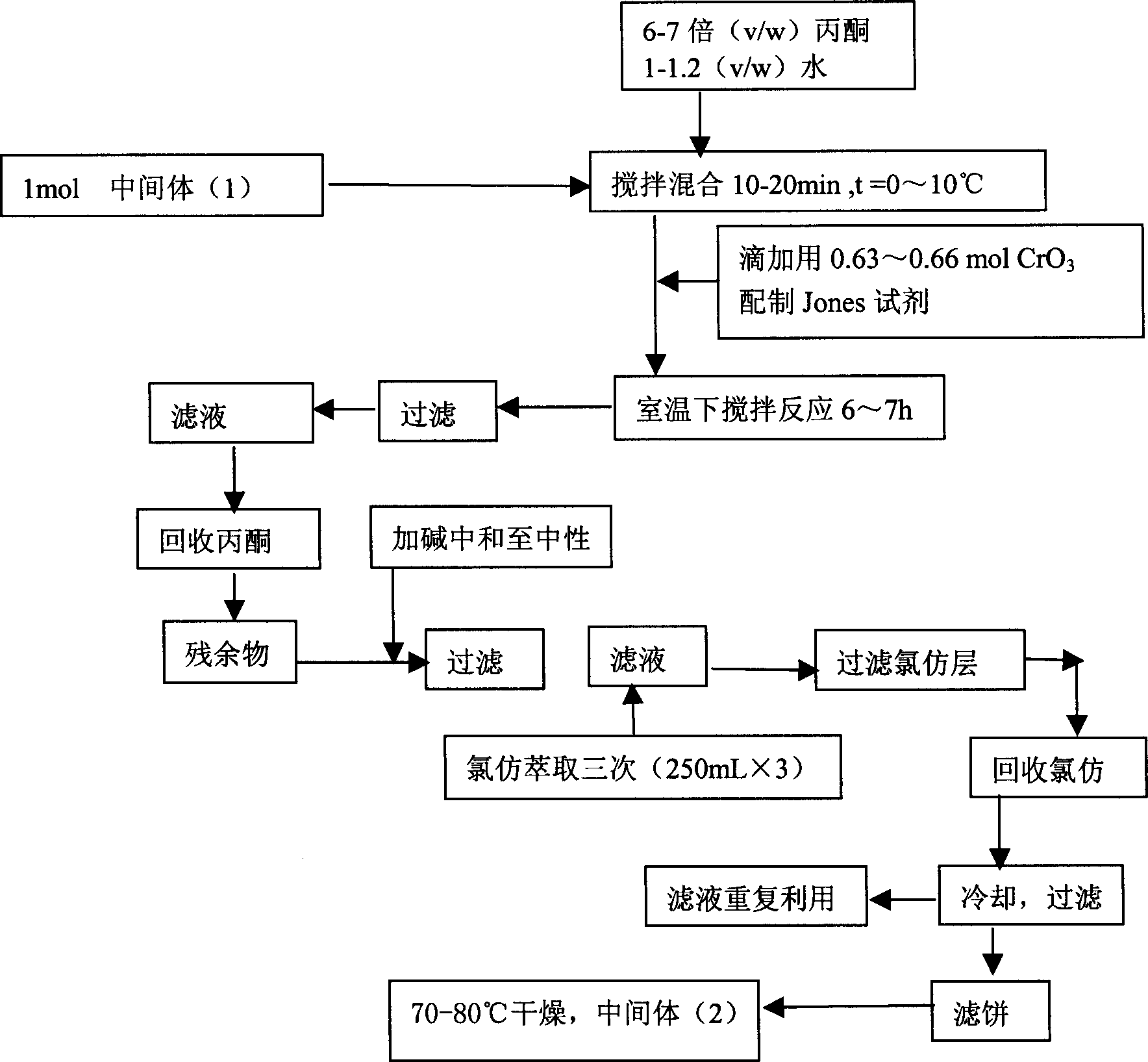
[0022] Second step reaction: Synthesis of 4-acetamidocyclohexanone (2)
[0023] Add 1 mol of the intermediate product (1) into the reaction flask, then add 6 to 7 times (V / W) acetone and 1 to 1.2 times (V / W) water, stir for 10 to 20 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com