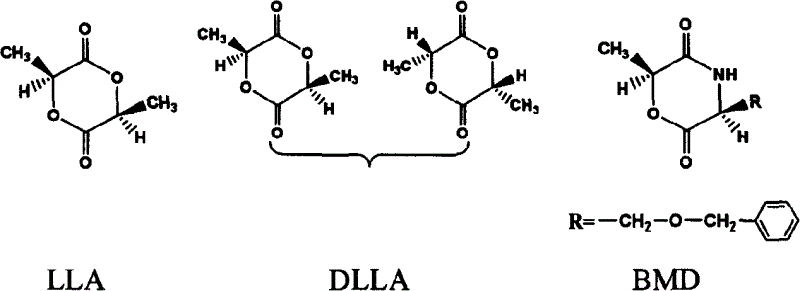
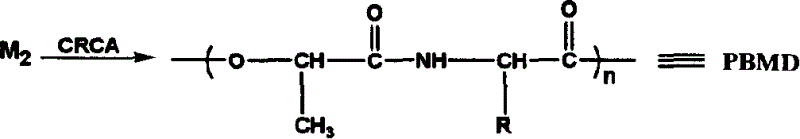
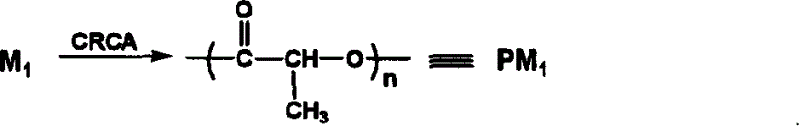Catalyzing synthesis of polylactide and polyserine morphodilone from carboxylic acid creatinine guanidine
A technology of polyserine morpholine dione and serine morpholine dione, which is applied in the field of catalytic synthesis of medical biodegradable polyester, can solve problems such as unsafe hidden dangers and cytotoxicity, and achieve no environmental pollutants and simple process , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Creatinine acetate catalyzed synthesis of poly-L-lactide
[0027] Weigh 1.728g (0.012mol) of L-lactide and 0.0069g (0.00004mol) of guanidine acetate creatinine respectively and put them in the reactor. Reacted in the bath for 96 hours. After the reaction, the polymer was dissolved with a small amount of acetone, slowly dropped into distilled water to precipitate the polymer, filtered to remove the distilled water, and the obtained solid was vacuum-dried for 24 hours. Yield: 94.0%, Mn=4.0×10 4 , PDI = 1.08.
Embodiment 2
[0028] Example 2 Creatinine acetate catalyzed synthesis of poly-L-lactide
[0029]Weigh 1.728g (0.012mol) of L-lactide and 0.0069g (0.00004mol) of guanidine acetate creatinine respectively and put them in the reactor. Reacted in the bath for 96 hours. After the reaction, the polymer was dissolved with a small amount of acetone, slowly dropped into distilled water to precipitate the polymer, filtered to remove the distilled water, and the obtained solid was vacuum-dried for 24 hours. Yield: 96.1%, Mn=4.2×10 4 , PDI = 1.10.
Embodiment 3
[0030] Example 3 Creatinine acetate catalyzed synthesis of poly-L-lactide
[0031] Weigh 1.728g (0.012mol) of L-lactide and 0.0069g (0.00004mol) of guanidine acetate creatinine respectively and put them in the reactor. Reacted in the bath for 96 hours. After the reaction, the polymer was dissolved with a small amount of acetone, slowly dropped into distilled water to precipitate the polymer, filtered to remove the distilled water, and the obtained solid was vacuum-dried for 24 hours. Yield: 92.8%, Mn=4.0×10 4 , PDI = 1.19.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com