Catalytic decomposition hydrogen release method for fullerene multihydrid hydrogen-storage material
A hydrogen storage material, catalytic decomposition technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, hydrogen, etc., can solve the problems of difficult processing, no research, high cost, etc., and achieve high hydrogen storage density Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Thermocatalytic C 60 h 36 split hydrogen
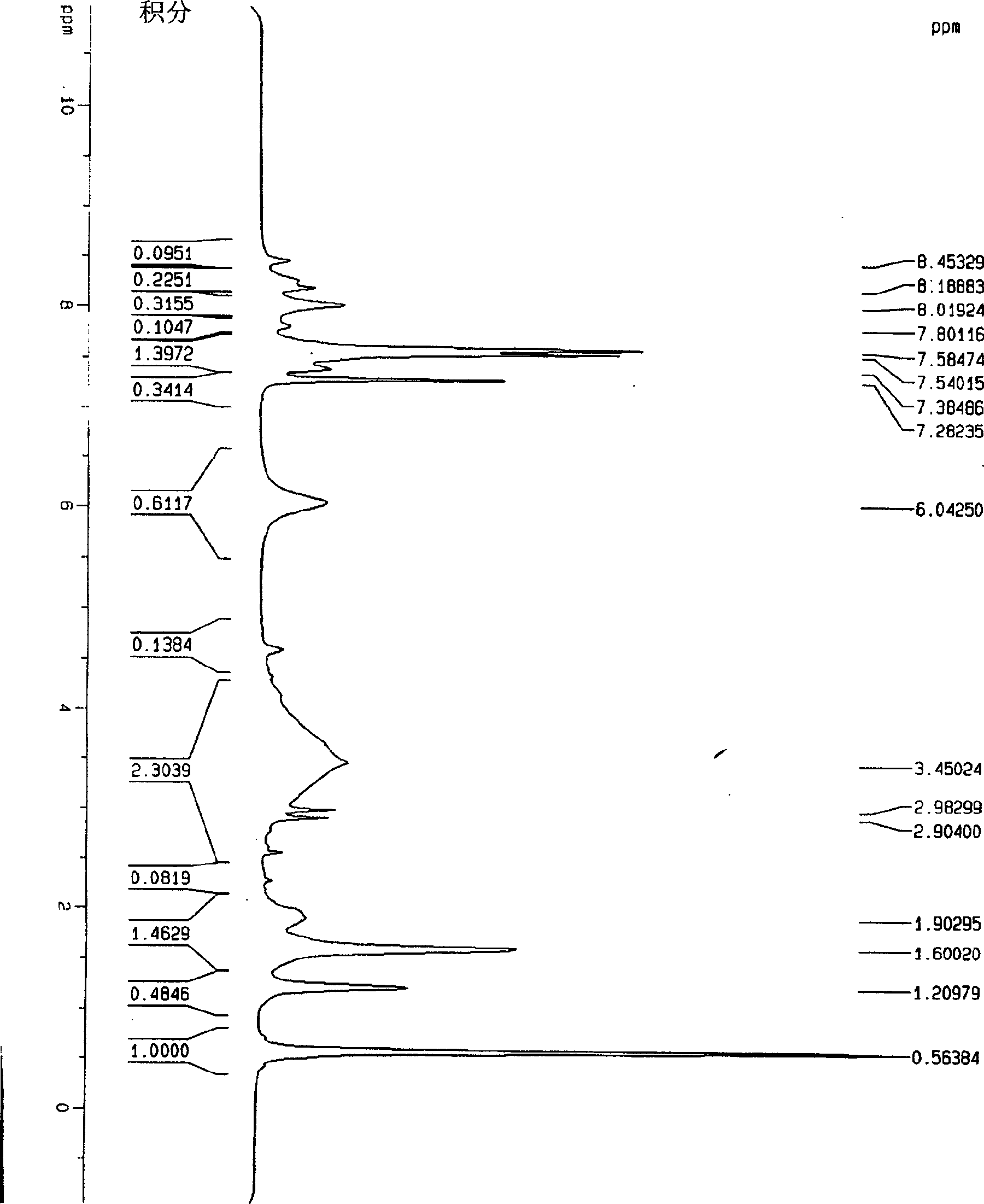
[0039] 20 mg C 60 h 36 , 15 mg Ir(CO)(PPh 3 ) 2 Cl and 0.5ml d 4 -Deuterated o-dichlorobenzene is added to the nuclear magnetic tube, then the nuclear magnetic tube is frozen in liquid nitrogen to solidify the reactant, the nuclear magnetic tube is evacuated with a vacuum pump, and then the vacuum pump is turned off, so that the solidified liquid in the nuclear magnetic tube is under vacuum Slowly heat and completely dissolve, then put the NMR tube into liquid nitrogen and freeze it. After repeating this operation 3 times, seal the tube, and then react at 60°C for 3 hours, hydrogen gas will be generated, and then measure the H-NMR spectrum and NMR spectrum. The place where δ=6.0 on the figure is H 2 peak, see figure 1 .
Embodiment 2
[0041] Thermocatalytic C 60 h 36 split hydrogen
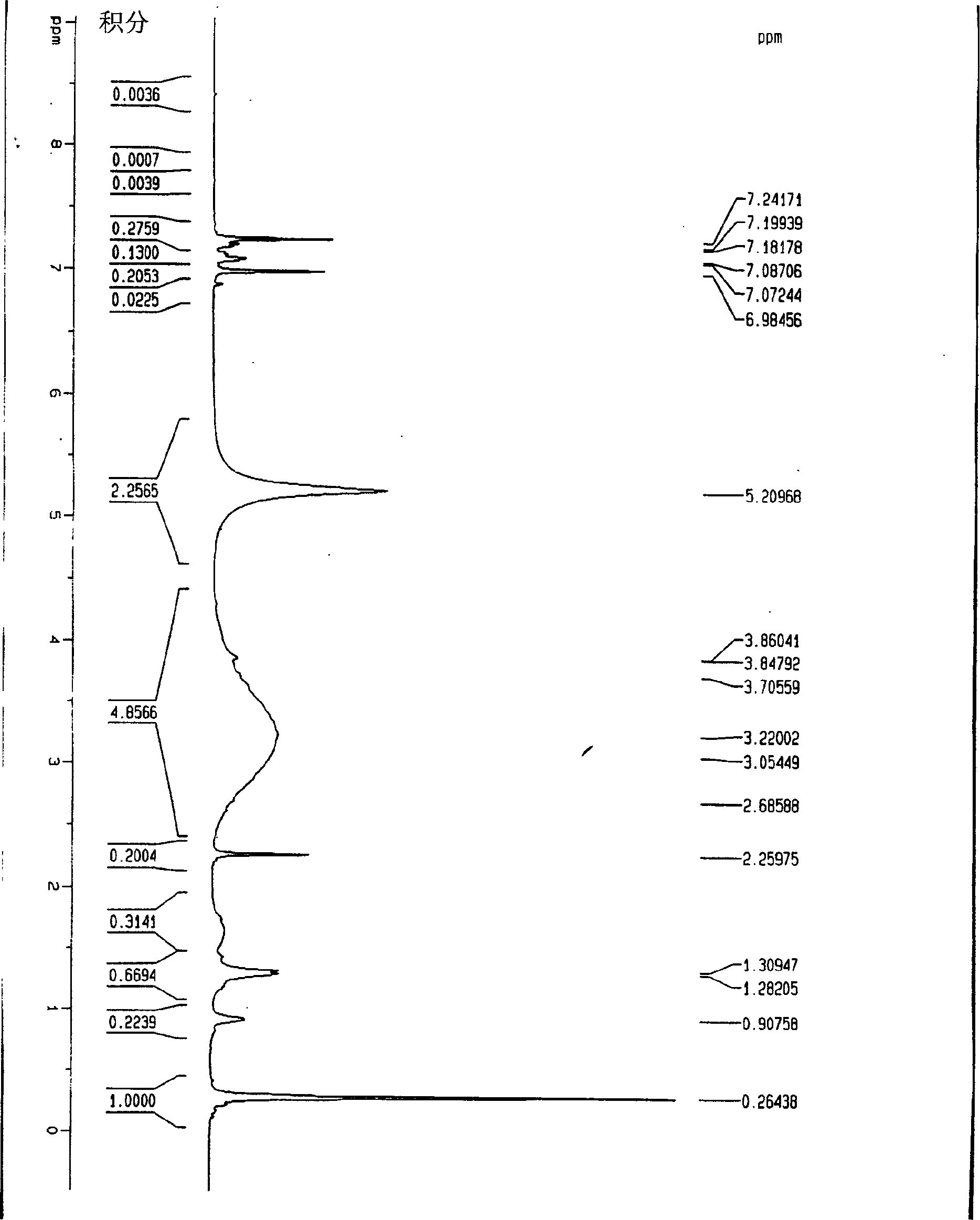
[0042] 20 mg C 60 h 36 , 10 mg of Pd / C (wherein the mass percentage of Pd is 10%) and 0.5 ml of d 4 -Deuterated o-dichlorobenzene is added to the nuclear magnetic tube, then the nuclear magnetic tube is frozen in liquid nitrogen to solidify the reactant, the nuclear magnetic tube is evacuated with a vacuum pump, and then the vacuum pump is turned off, so that the solidified liquid in the nuclear magnetic tube is under vacuum Slowly heat and completely dissolve, then put the NMR tube into liquid nitrogen and freeze it. After repeating this operation 3 times, seal the tube, and then react at 100°C for 16 hours, hydrogen gas will be generated, and then measure the H-NMR spectrum and NMR spectrum. The place where δ=5.2 on the figure is H 2 peak, see figure 2 .
Embodiment 3
[0044] photocatalytic C 60 h 36 split hydrogen
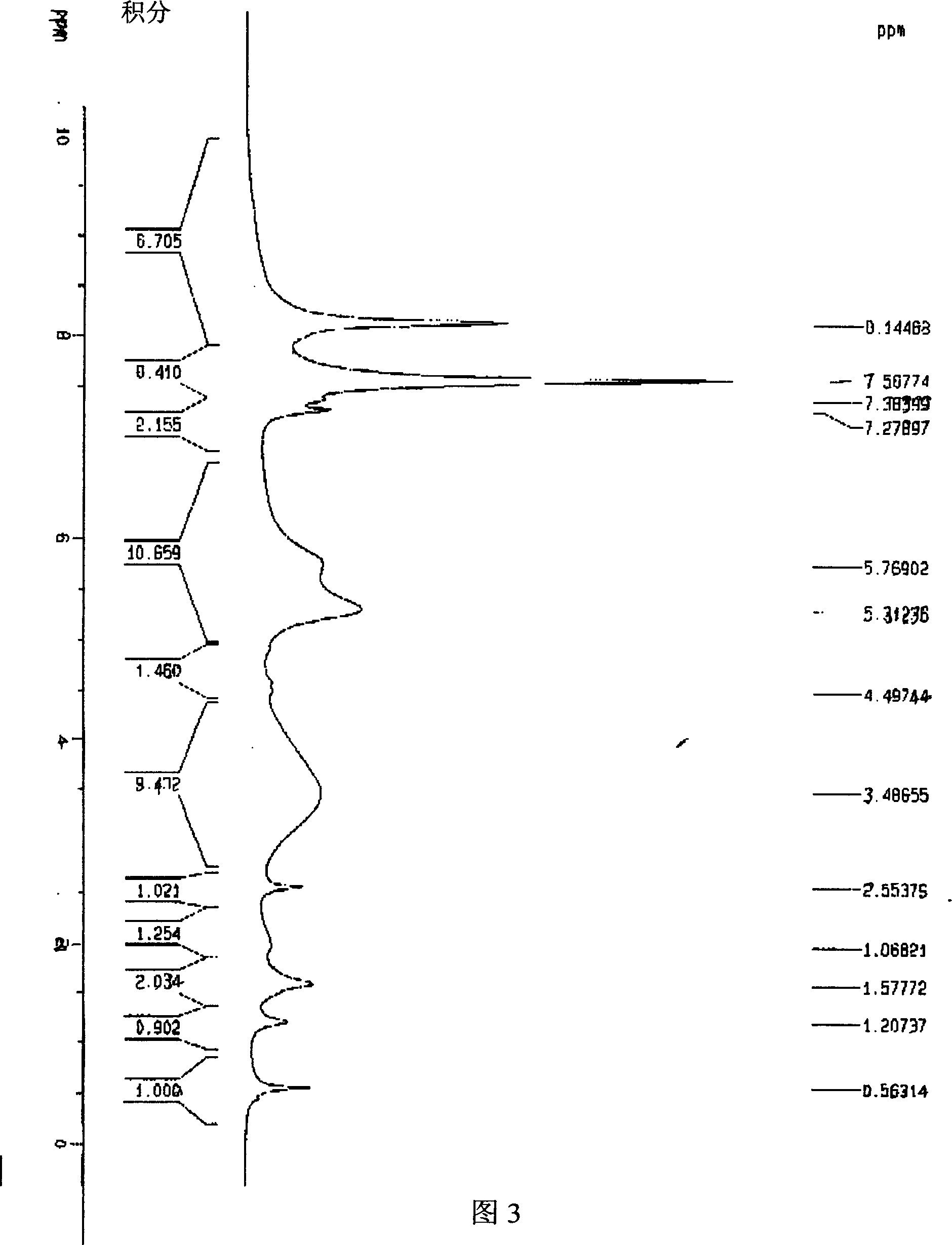
[0045] 20 mg C 60 h 36 , 10 mg Rh(CO)(PPh 3 ) 2 Cl and 0.5ml d 4 -Deuterated o-dichlorobenzene is added to the nuclear magnetic tube, then the nuclear magnetic tube is frozen in liquid nitrogen to solidify the reactant, the nuclear magnetic tube is evacuated with a vacuum pump, and then the vacuum pump is turned off, so that the solidified liquid in the nuclear magnetic tube is under vacuum Slowly heat and completely dissolve, then put the NMR tube into liquid nitrogen and freeze it. After repeating this operation for 3 times, seal the tube, and then react under 365nm ultraviolet light for 6 hours, then hydrogen gas will be generated, and then measure the H NMR spectrum. On the NMR spectrum, δ=5.3~5.8 is H 2 peak, see Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com