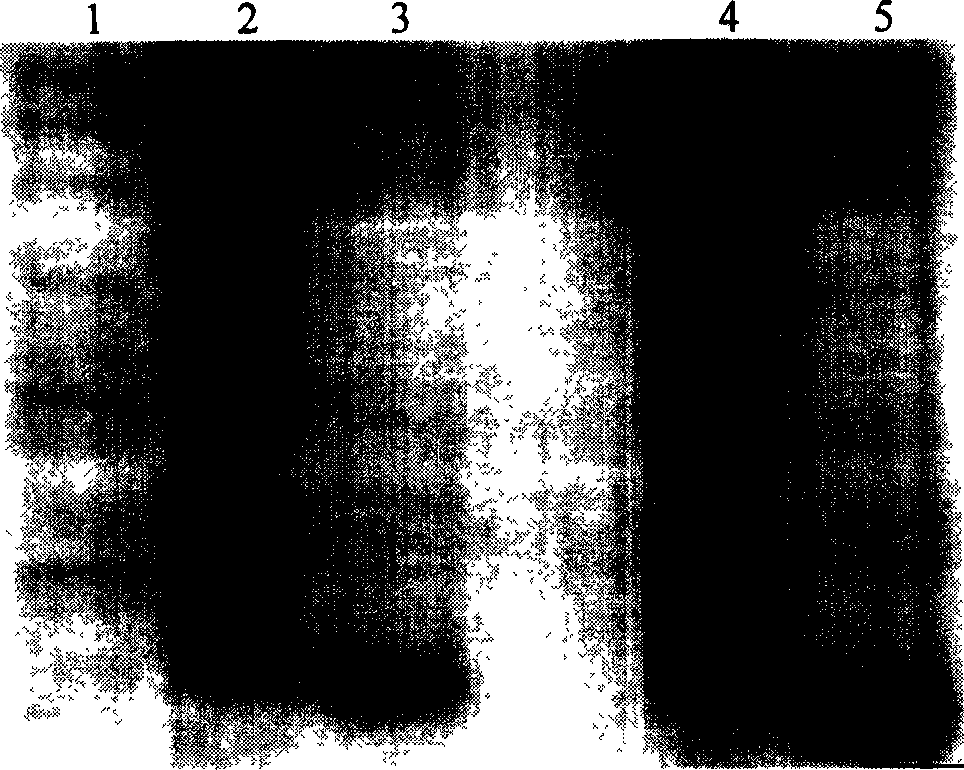Fusion protein of tumour new-born blood vessel-specific bonding polypeptide and recombinant human Tum-5, and its preparation method
A technology of tumor neovascularization and fusion protein, which is applied in the field of tumor neovascularization-specific binding polypeptide and recombinant human Tum-5 fusion protein and its preparation, which can solve the problem of no anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
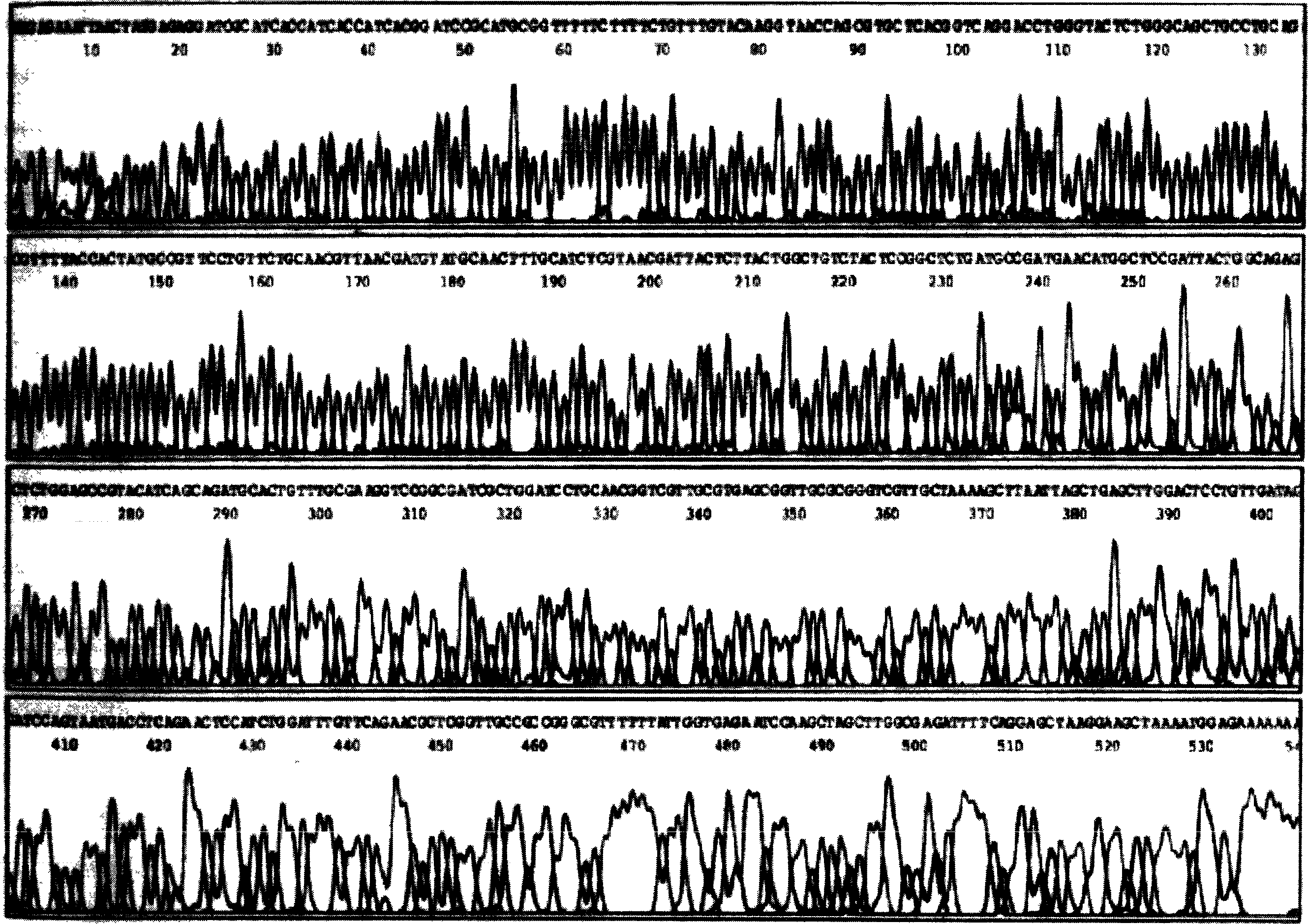
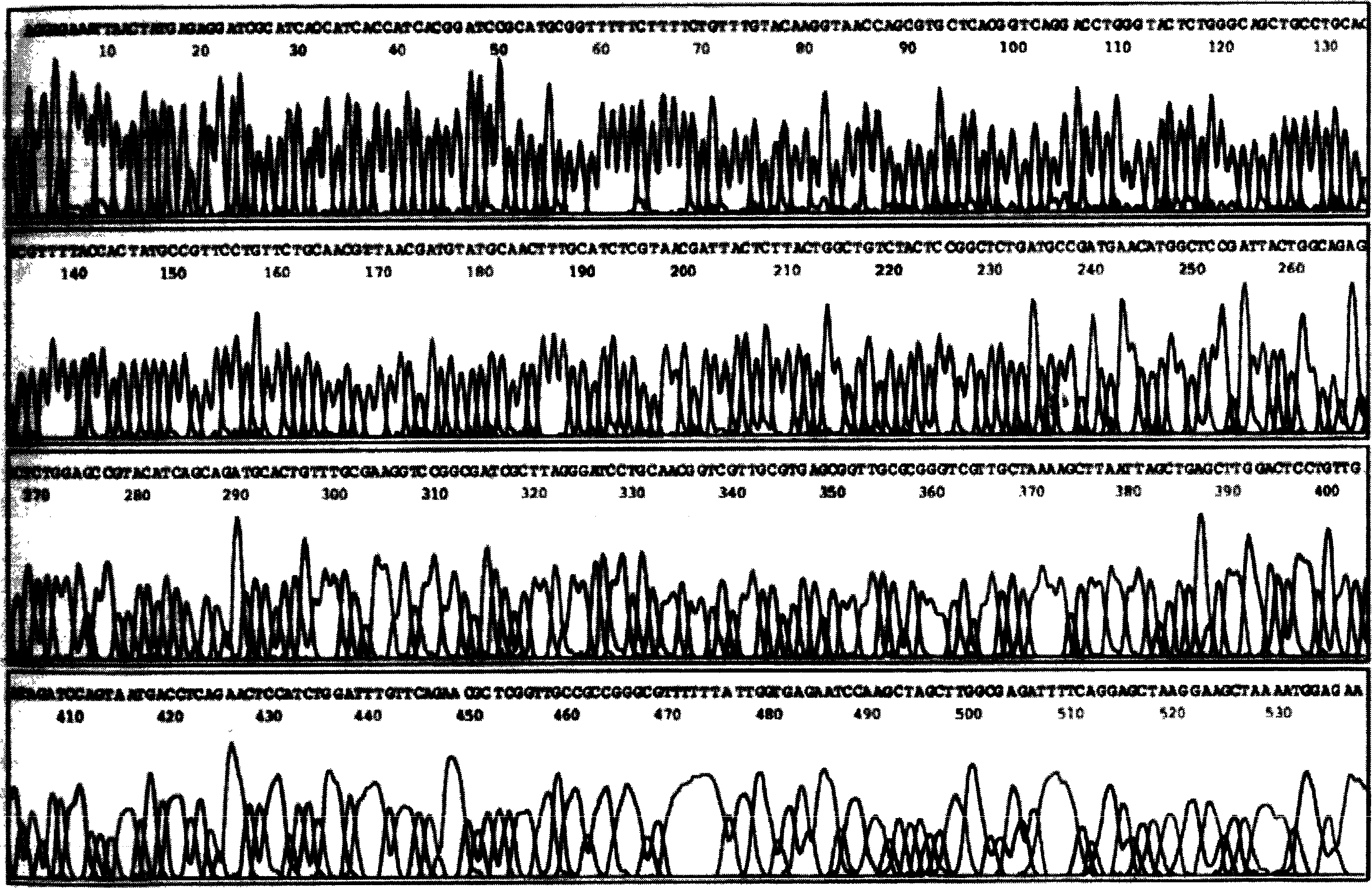
[0056] 1. Construction of Tum-5 and Tum-5-NGR His fusion gene prokaryotic expression vector
[0057] 1.1 Construction of prokaryotic expression vector of Tum-5His fusion gene
[0058] According to the human Tum-5 gene sequence published in GenBank, the codons encoding Tum-5 amino acids were replaced with codons commonly used in Escherichia coli (which is conducive to the high-efficiency expression of the target protein in Escherichia coli), and the codon was handed over to Shanghai Boya Biotechnology Co., Ltd. The company synthesized the whole gene, introduced the SphI restriction site upstream and the HindIII restriction site downstream, and cloned the target gene into the vector pMD-18T, named pMD-18T-Tum-5.
[0059] After pMD-18T-Tum-5 was double-digested with SphI and HindIII, small fragments were recovered; after plasmid pQE30 (product of Qiagen Company) was double-digested with SphI and BamH1, large fragments were recovered. The above two fragments were ligated with T4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com