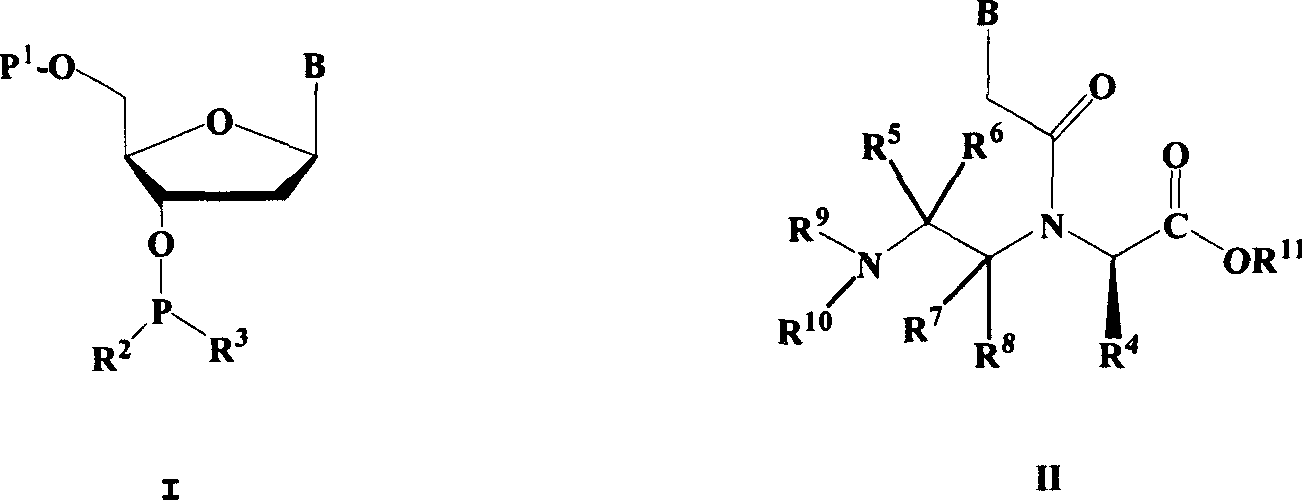
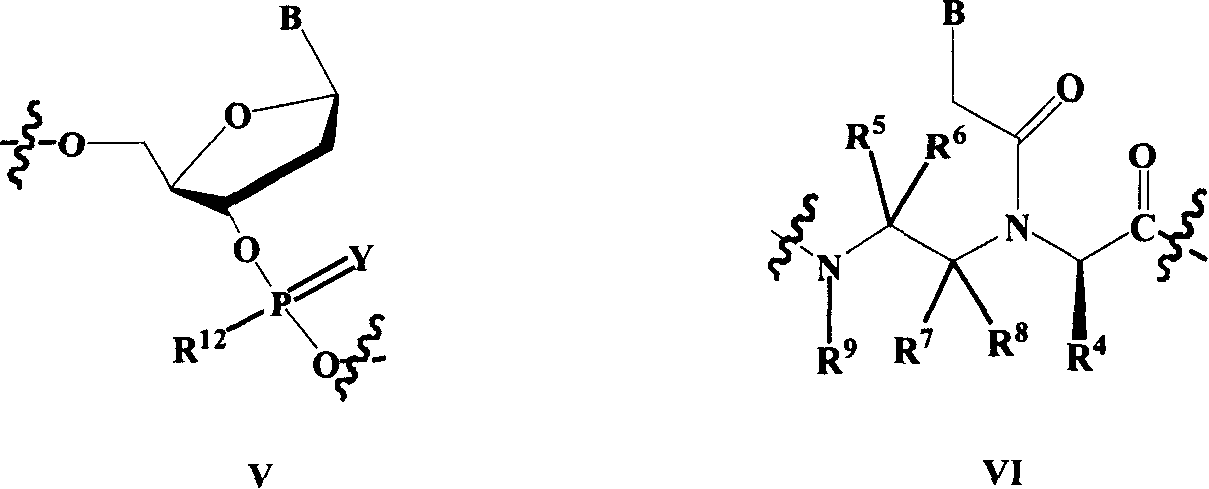Nucleic acid, peptide nucleicacid derivatives and their use
A technology of peptide nucleic acid and nucleoside analogs, which is applied in the field of nucleic acid and peptide nucleic acid derivatives, and can solve problems such as failure to reach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] 1.1 1-(2-deoxy-3,5-di-oxo-p-methylbenzoyl-β-D-erythro-ribose)-5-(2-hydroxyethyl)-1H,3H-pyrimidine-2 , 4-diketone (1)
[0108] Suspend 5-hydroxyethyluracil (4g, 25.64mmol) in hexamethyldisilazane (20ml), add a small amount of ammonium sulfate, heat the mixture, and reflux overnight. After the remaining hexamethyldisilazane was distilled off under reduced pressure, chloroform (400ml) was added, followed by 1-chloro-2-deoxy-3,5-di-p-methylbenzoyl ribose (12g, 30.89mmol) and cuprous iodide (5.9 g, 30.89 mmol), stirred at room temperature for 3 hours. The mixture was poured into a saturated sodium bicarbonate solution, stirred for 1 h, the organic layer was separated, washed once with water, dried with anhydrous sodium sulfate, filtered and concentrated to obtain a syrupy liquid, which was separated by column chromatography as a colorless solid product. 11.5 g, yield 88.5%. R f (CH 2 Cl 2 / CH 3 OH 20:1) 0.35. 1 H NMR (d6-DMSO): 2.26(t, J=6.7Hz, 5-CH 2 CH 2 O), 2.39...
Embodiment 2
[0119] 2.15-(3-tert-butyldiphenylsiloxypropyl)-uracil (6)
[0120] According to the method of Example 1.3, in dichloromethane (20ml), with 5-(3-trihydroxypropyl)uracil (1.5g, 8.81mmol), imidazole (0.66g, 9.7mmol), tert-butyldiphenyl Chlorosilane (2.56ml, 9.8mmol) gave the product 3-tert-butyldiphenylsiloxypropyluracil, 3.4g, yield 94.4%. R f (CH 2 Cl 2 / CH 3 OH 20:1) 0.15. Elemental Analysis: C 23 h 28 N 2 o 3 Si (M 408.57), theoretical: C 67.61, H 6.91, N 6.86; found: C 67.56, H 6.78, N 6.74. 1 H NMR (d6-DMSO): 0.99 (s, tBu), 1.69 (m, 5-CH 2 CH 2 CH 2 ), 2.25(t, J=7.3Hz, 5-CH 2 CH 2 CH 2 ), 3.62(t, J=6.3Hz, 5-CH 2 CH 2 CH 2 ), 7.15 (s, 6-H), 7.45, 7.61 (2m, Ph), 11.01, 10.63 (s, 2NH).
[0121] 2.2 1-(2-Deoxy-3,5-di-oxo-p-methylbenzoyl-β-D-erythro-ribose)-5-(2-diphenyltert-butylsiloxypropyl)- 1H,3H-pyrimidine-2,4-dione (7)
[0122] According to the method of Example 1.1, after 5-(3-tert-butyldiphenylsiloxypropyluracil (2.4g, 5.88mmol) was silanized with he...
Embodiment 3
[0131] 3.1 1-(β-D-erythro-2-deoxy-ribose)-1H,3H-pyrimidine-2,4-dione-5-propionic acid methyl ester (12)
[0132] It was prepared according to the synthesis method of compound 1. After obtaining compound 11, the deprotection reaction was carried out directly to obtain compound 12, 2.6 g, with a yield of 45.6%. R f (CH 2 Cl 2 / CH 3 OH 9:1) 0.42. Elemental Analysis: C 13 h 18 N 2 o 7 (M 314.29), Theoretical: C 49.68, H 5.77, N 8.91; Found: C 49.67, H 5.52, N 8.47. 1 H NMR (d6-DMSO): 2.08 (m, 2'-H), 2.48 (m, 5-CH 2 CH 2 ), 3.59 (s, CH 3 ), 3.56(m, 5'-H), 3.77(m, 4'H), 4.24(m, 3'-H), 5.03(t, J=5.2Hz, 5'-OH), 5.24(d, J = 4.2 Hz, 3'-OH), 6.16 (t, J = 6.9 Hz, 1'-H), 7.72 (s, 6-H), 11.33 (s, NH).
[0133] 3.2 1-(β-D-erythro-deoxy-ribose)-1H,3H-pyrimidine-2,4-dione 5-propionamide (13)
[0134] Ammonia / methanol solution (70 ml) of compound 12 (0.5 g, 1.59 mmol) was placed in a closed container and stirred at room temperature for 7 days. The reaction solution was concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com