Cation fluoride Gemini surface activator
A surfactant, cnf2n technology, applied in dissolution, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of surface activity and other unsatisfactory properties, and achieve low critical micelle concentration and good dispersibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
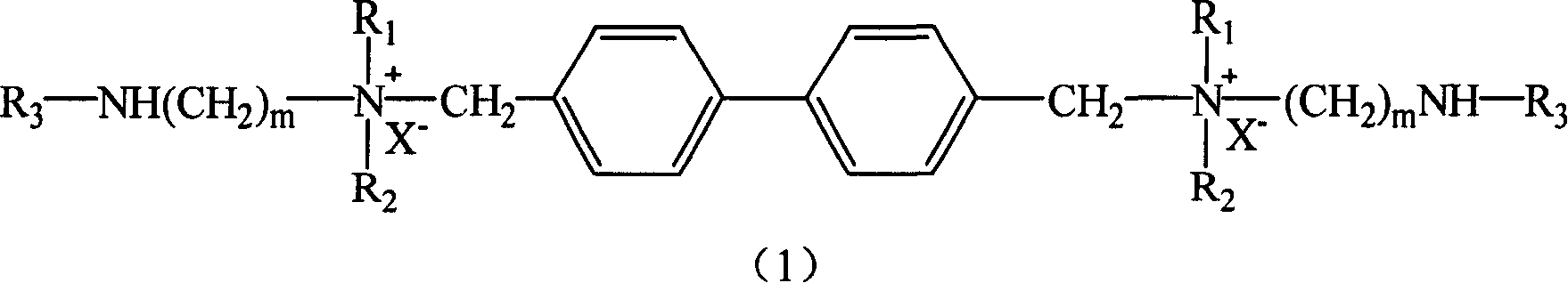
[0023] Synthesis of Surfactant (I):
[0024]
[0025] 1. Synthesis of perfluorosulfonamide:
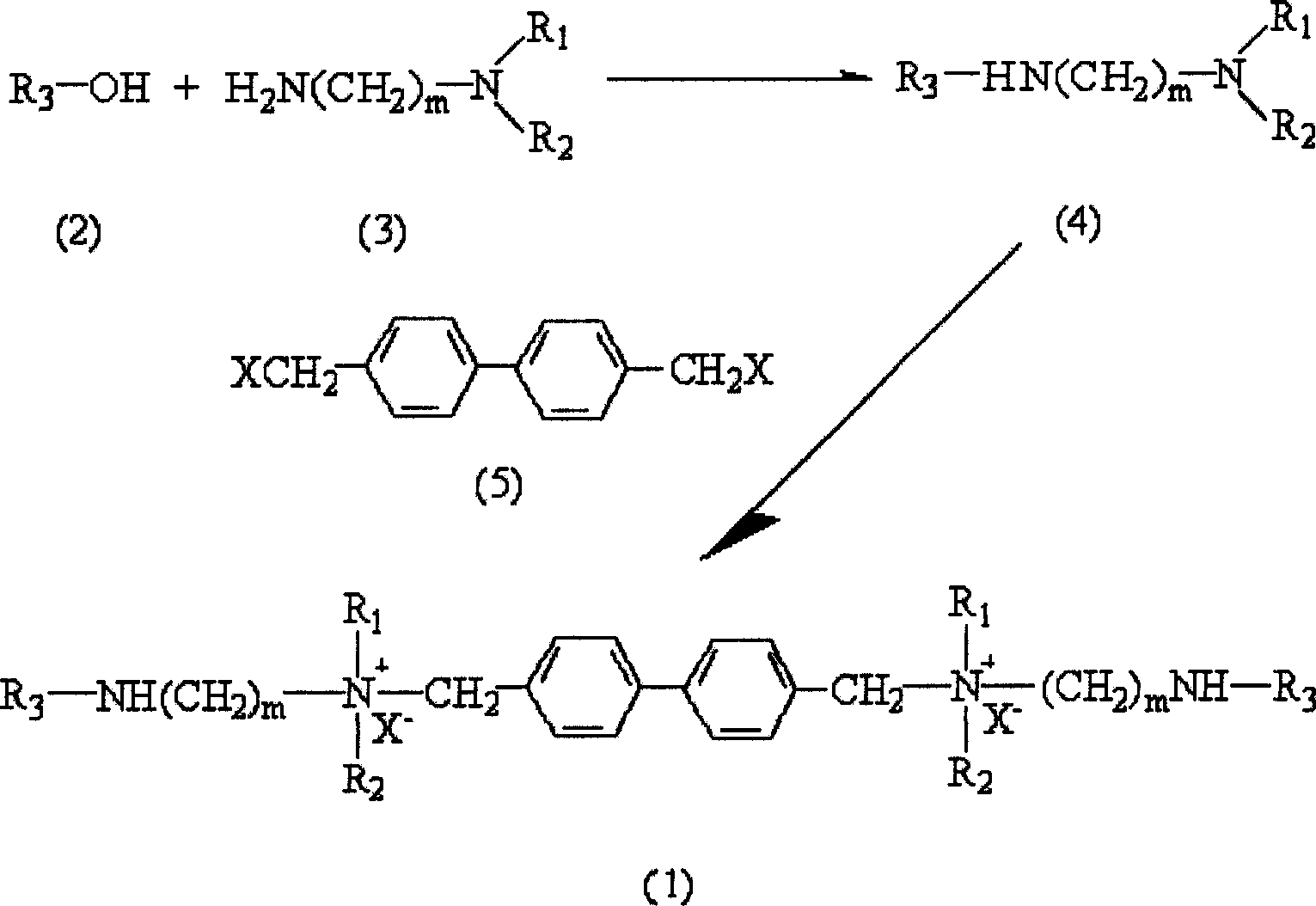
[0026] Add 100ml of toluene and 0.01mol of perfluorooctane sulfonic acid [the compound represented by formula (2) in a three-necked flask, where R 3 =C n F 2n+1 SO 2 , N=8], then slowly drop 0.02mol of amine [the compound represented by formula (3), where m=2, R 1 , R 2 Both are CH 3 ] Reflux for 12 hours at 110℃~120℃. After the reaction, the toluene was evaporated to dryness to obtain a crude product, which was recrystallized to obtain perfluorosulfonamide.
[0027] 2. Synthesis of Gemini Surfactant:
[0028] Add the previously prepared 0.01mol perfluorosulfonamide (prepared in step 1) and 50ml diethyl ether into a three-necked flask, then add 0.02mol 4,4'-dichloromethylbiphenyl, reflux and react for 48 hours to obtain a white solid It is a surfactant product.
[0029] 1 H NMR(DMSO-d 6 )δ(ppm): 1.91(s, 2H), 3.09(t, J=7.2Hz, 4H), 3.32(s, 12H), 3.50(t, J=5.7Hz, 4H), 4.49(s, 4H) , 7.12 (d...
Embodiment 2
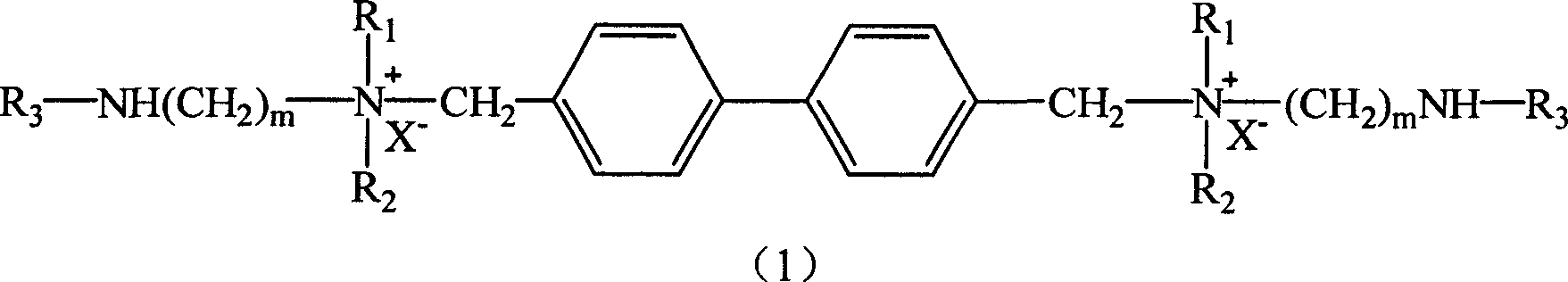
[0031] Synthesis of Surfactant (II):
[0032]
[0033] 1. Synthesis of perfluorosulfonamide:
[0034] Add 100ml of toluene and 0.01mol of perfluorooctane sulfonic acid [the compound represented by formula (2) in a three-necked flask, where R 3 =C n F 2n+1 SO 2 , N=6], then slowly add 0.02mol of amine [the compound represented by formula (3), where m=4, R 1 , R 2 All -C 2 H 5 ], the reaction was refluxed at 110℃~120℃ for 12 hours. After the reaction, the toluene was evaporated to dryness to obtain a crude product, which was recrystallized to obtain perfluorosulfonamide.
[0035] 2. Synthesis of Gemini Surfactant:
[0036] Add 0.01mol of perfluorosulfonamide prepared in step 1 and 50ml of ether into a three-necked flask, then add 0.02mol of 4,4'-dichloromethylbiphenyl, and react under reflux for 48 hours. The white solid obtained is surface active.剂产品。 Agent products.
[0037] 1 H NMR(DMSO-d 6 )δ(ppm): 0.89(t, J=7.8Hz, 12H), 1.41~1.47(m, 4H), 1.77(m, 4H), 1.81(s, 2H), 2.65(t, 4H),...
Embodiment 3
[0040] Synthesis of Surfactant (III):
[0041]
[0042] 1. Synthesis of perfluorooctamide:
[0043] Add 100ml of toluene and 0.01mol of perfluorooctanoic acid [the compound represented by formula (2) in a three-necked flask, where R 3 =C nF 2n+1 CO, n=7], then slowly add 0.02mol of amine [the compound represented by formula (3), where m=4, R 1 , R 2 All -CH 3 ], reflux for 12 hours at 110°C to 120°C. After the reaction, the toluene was evaporated to dryness to obtain a crude product, which was recrystallized to obtain perfluorooctylamide.
[0044] 2. Synthesis of Gemini Surfactant:
[0045] In a three-necked flask, 0.01 mol of perfluorooctamide and 50 ml of ethyl ether were added, and then 0.02 mol of 4,4'-dibromomethyl biphenyl was added, and the reaction was refluxed for 48 hours. The white solid obtained was the surfactant product.
[0046] 1 H NMR(DMSO-d 6 )δ(ppm): 1.47~1.53(m,4H), 1.70~1.79(m,4H), 3.20(m,4H), 3.26(t,J=5.7Hz,4H), 3.41(s,12H), 4.51 (s, 4H), 7.11 (d, J=8.5 Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com