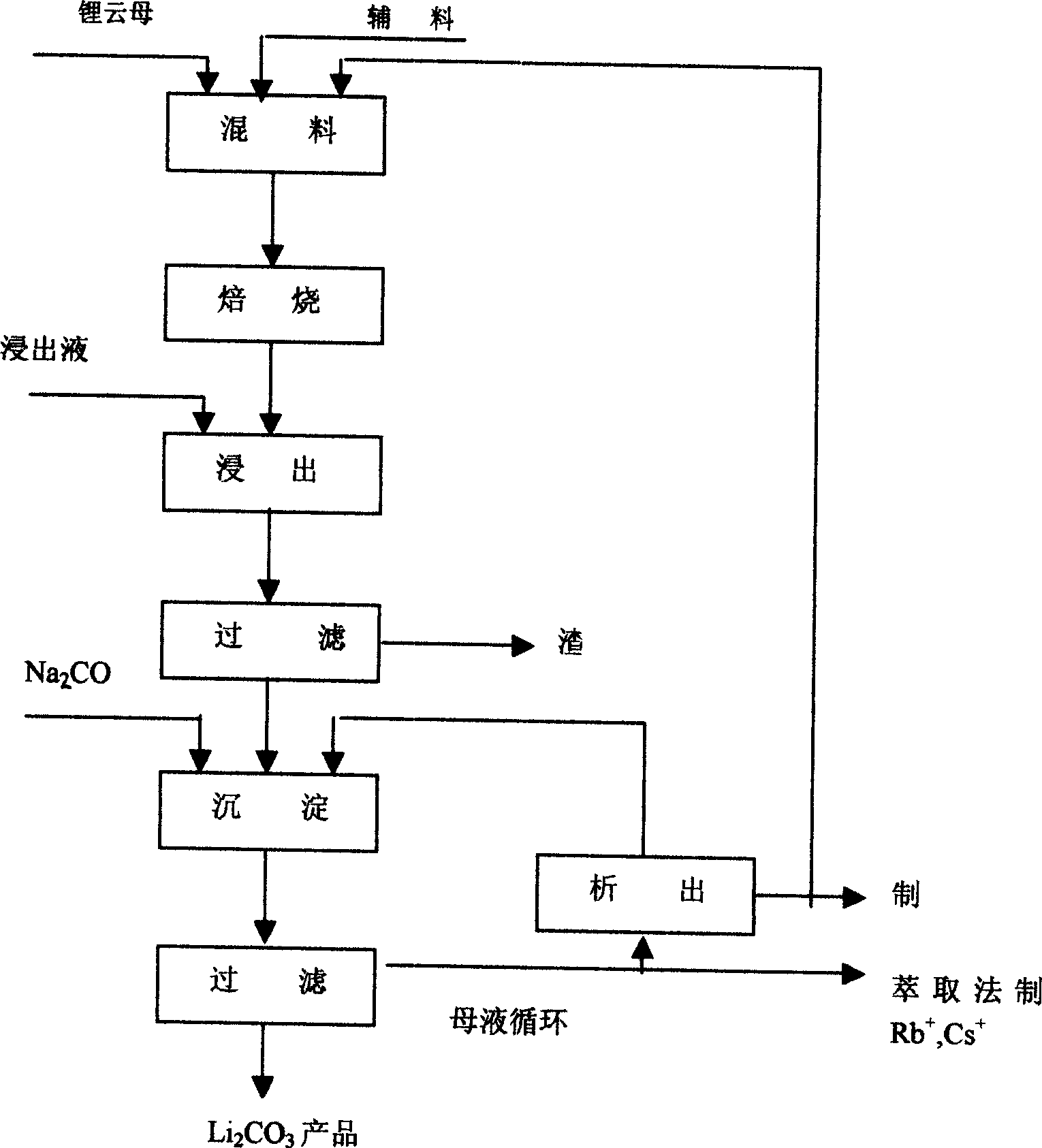
Process for preparing lithium chlorate by lithium extracted from lepidolite
A lepidolite and part of the technology is applied in the field of extracting lithium to produce lithium carbonate, which can solve the problems of difficult industrial implementation, high equipment requirements, and large consumption of potassium sulfate, so as to solve the problem of equipment anticorrosion, reduce the evaporation and concentration process, and save H2SO4. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] With lepidolite ore (composition is shown in Table 1) 1000 grams and industrial grade Na 2 SO 4 , Industrial Grade CaF 2 According to the mass ratio of 1:0.2:0.3, the ingredients are ball milled and dry mixed in a ball mill, and roasted in a tubular resistance furnace. The firing temperature is 900°C. Roasting time is 60 minutes, take out and cool, grind to 60-80 mesh with ball mill,
[0019] Li 2 o
Embodiment 2
[0021] With the lepidolite ore 1000g of table 1 composition, and industrial grade CaSO 4 2H 2 O, industrial grade CaO is batched according to mass ratio 1: 0.4: 0.2, carries out ball milling, dry mixing in ball mill, follow-up process is the same as embodiment 1, and the results are shown in Table 2.
Embodiment 3
[0023] With the lepidolite ore 1000g of table 1 composition, and industrial grade CaSO 4 2H 2 O, industrial grade Na 2 SO 4 According to the mass ratio of 1: 0.4: 0.4 ingredients, ball milling and dry mixing were carried out in a ball mill, and the follow-up process was the same as in Example 1. The results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com