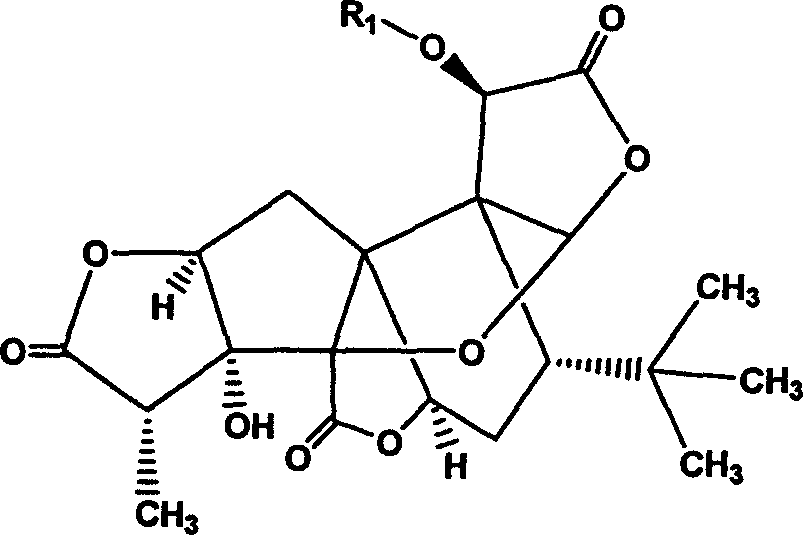
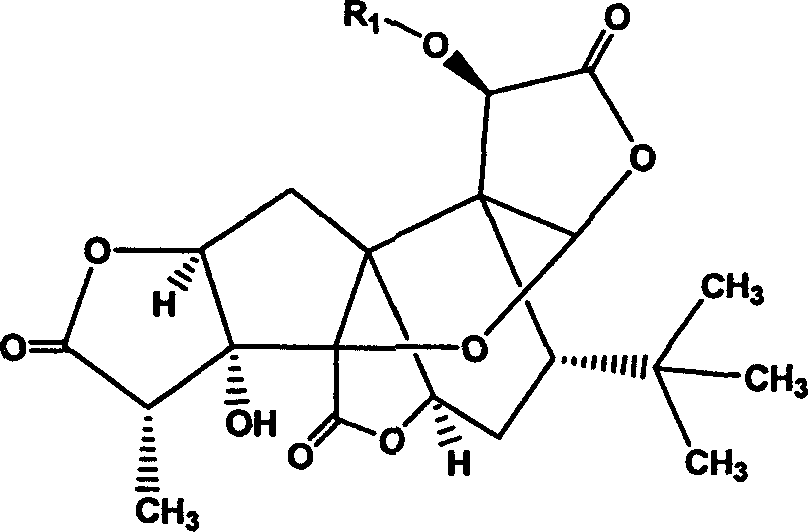
Bilobalide A derivatives and pharmaceutical application thereof
A kind of technology of ginkgolide and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Example 1. Preparation and structure confirmation of 10-(2'-dimethylaminoethoxy)-ginkgolide A
[0165] Dissolve 600mg of ginkgolide A in 40mL of acetonitrile, add 300mg of N,N-dimethylchloroethylamine, 2.5g of potassium carbonate, and 300mg of potassium iodide in sequence, pass inert gas, heat and reflux for 2h, concentrate under reduced pressure, dissolve in chloroform, filter, The filtrate was concentrated. The obtained product was separated by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 1) to obtain 670 mg of the expected product (yield 95.2%).
[0166] 1 H-NMR (DMSO-d 6 )δ6.31(s, 1H), 6.05(s, 1H), 5.31(d, 1H), 5.11(s, 1H), 4.55(d, 1H), 4.30(t, 1H), 3.50(t, 1H ), 2.81(q, 1H), 2.60(d, 2H), 2.30(d, 2H), 2.15(s, 6H), 2.13(dd, 1H), 1.86(ddd, 1H), 1.60(dd, 1H) , 1.10(d, 3H), 1.05(s, 9H).
Embodiment 2
[0167] Example 2. Preparation and structure confirmation of 10-(2'-diethylaminoethoxy)-ginkgolide A
[0168] Dissolve 600mg of ginkgolide A in 40mL of acetonitrile, add N,N-diethylchloroethylamine 350mg, potassium carbonate 2.5g, potassium iodide 300mg, pass inert gas, and heat to reflux for 2h. Concentrate under reduced pressure, dissolve in chloroform, filter, and concentrate the filtrate. The obtained product was separated by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 1) to obtain 705 mg of the expected product (yield 94.2%).
[0169] 1 H-NMR (DMSO-d 6 )δ6.20(s, 1H), 6.07(s, 1H), 5.26(d, 1H), 5.14(s, 1H), 4.65(d, 1H), 4.30(t, 1H), 1.95(d, 1H ), 3.52(t, 1H), 2.84(q, 1H), 2.60(d, 2H), 2.5(s, 4H), 2.30(d, 2H), 2.15(s, 6H), 1.88(ddd, 1H) , 1.76 (dd, 1H), 1.21 (d, 3H), 1.10 (s, 9H).
Embodiment 3
[0170] Example 3. Preparation and Structure Confirmation of 10-Benzyloxy-Ginkgolide A
[0171] Dissolve 600mg of ginkgolide A in 40mL of acetonitrile, add 500mg of benzyl bromide, 2.5g of potassium carbonate in sequence, pass inert gas, and heat to reflux for 2h. Concentrate under reduced pressure, dissolve in chloroform, filter, and concentrate the filtrate. The obtained product was separated by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 1) to obtain 705 mg of the expected product (yield 96.1%).
[0172] 1 H-NMR (CDCl 3 )δ7.28~7.11(m, 5H), 6.34(s, 1H), 6.05(s, 1H), 5.18(brs, 1H), 5.25(s, 1H), 5.02(ABq, 2H), 4.60(d , 1H), 4.17(dd, 1H), 2.87(q, 1H), 2.71(d, 1H), 2.24(dd, 1H), 2.03(dd, 1H), 1.86(td, 1H), 1.73(dd, 1H), 1.15(d, 1H), 1.01(s, 9H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com