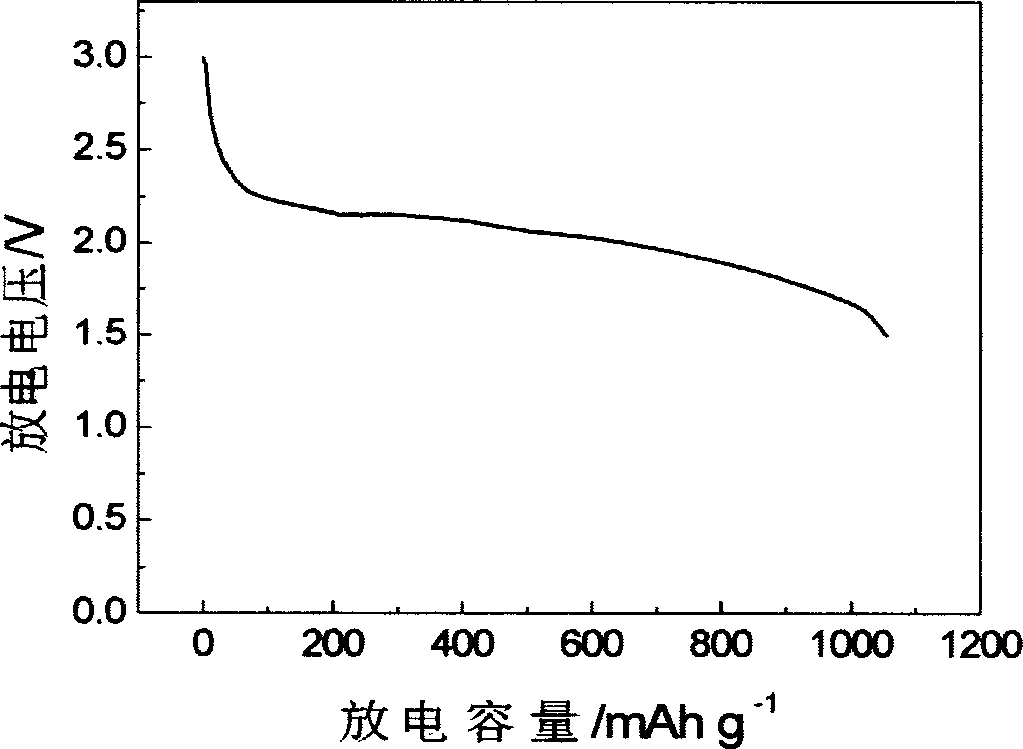
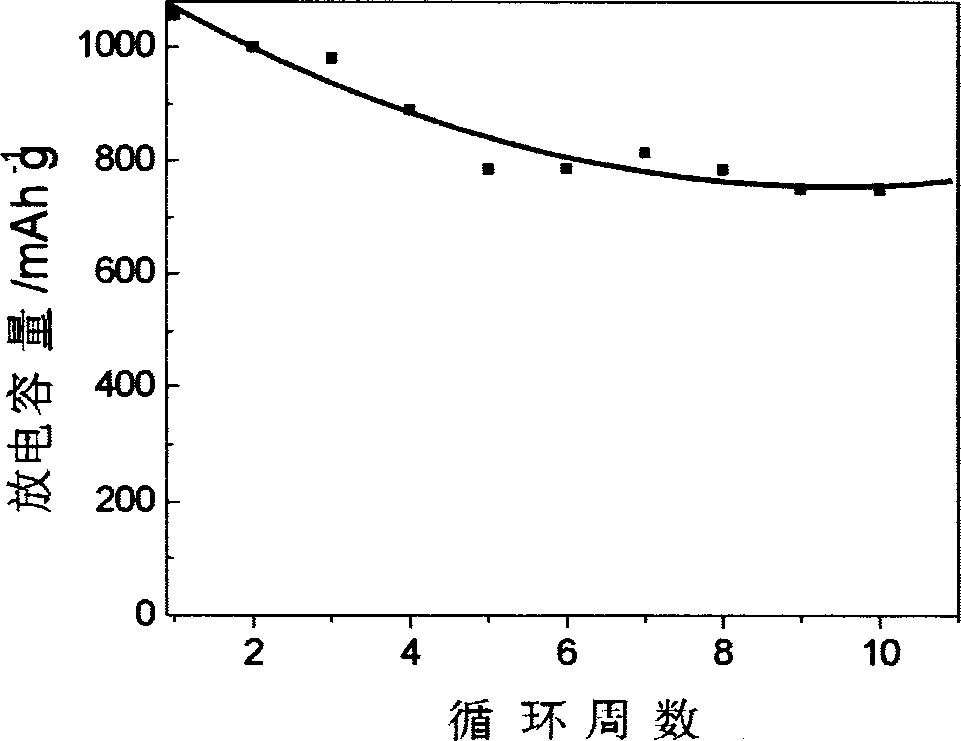
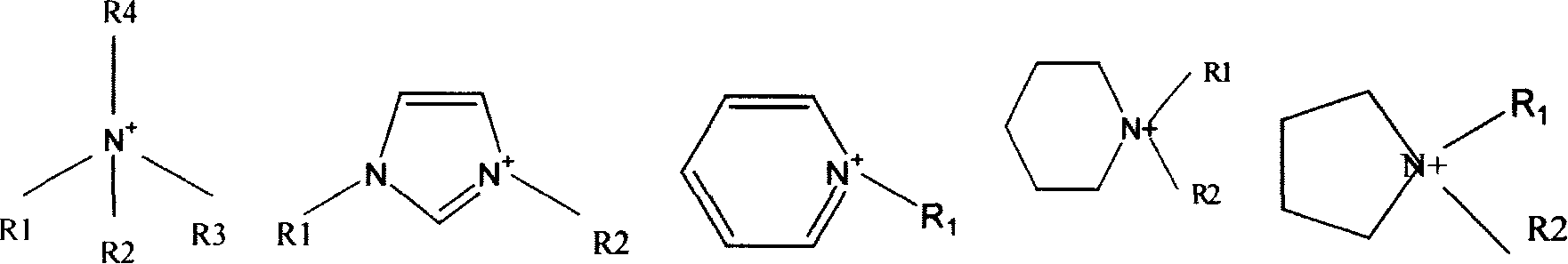
Lithium-sulphur battery electrolyte and chargeable lithium-sulphur battery thereof
An electrolyte, sulfur battery technology, applied in the field of electrochemical and chemical power products, can solve the problems of low utilization rate of active materials, poor battery cycle performance, reduced battery capacity, etc., to achieve excellent safety performance, long cycle life, specific capacity big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] With (EMIM)BF 4 (EMIM represents N-ethyl-N' methylimidazolium ion) as an example: the first step is quaternization, using methylimidazole (MIM) to prepare halogen-containing imidazolium salts, the reaction is as follows: . Specific operation: 35.0ml (0.63mol) of N-methylimidazole was vacuum distilled through KOH into a 250ml round bottom flask, and 90ml (0.69mol) of bromoethane was vacuum distilled through P 2 o 5 was added, the reaction mixture was refluxed for 2h under a nitrogen atmosphere, and cooled overnight to obtain a white solid dissolved in 50ml
[0048] In hot acetonitrile, filter under a nitrogen atmosphere, add 100ml of ethyl acetate to the filtrate, cool to -13°C, filter to obtain a precipitate, crystallize in 20ml of acetonitrile, filter, and dry in vacuum for 36h to obtain [EMIM]Br69.3g (yield 57.6%).
[0049] The second step is ion exchange, in which the halogen ions are exchanged for the required negative ions to obtain the ionic liquid. The ...
Embodiment 2
[0053] Take (EMIM)TFSI (where EMIM represents N-ethyl-N'methylimidazolium ion, TFSI represents N(CF 3 SO 2 ) 2 - ) is an example: the first step is quaternization, and the imidazolium salt containing halogen is obtained with methylimidazole, and the reaction is as follows:
[0054] Specific operation: 35.0ml (0.63mol) of N-methylimidazole vacuum distillation through KOH into a 250ML round bottom flask, 90ml (0.69mol) of bromoethane vacuum distillation through P 2 o 5 After adding, the reaction mixture was refluxed for 2 hours under a nitrogen atmosphere, and cooled overnight to obtain a white solid dissolved in 50ml of hot acetonitrile, filtered under a nitrogen atmosphere, 100ml of ethyl acetate was added to the filtrate, cooled to -13°C, and a precipitate was obtained by filtration. Crystallize in acetonitrile, filter, and dry in vacuum for 36 h to obtain [EMIM]Br69.3g (57.6% yield).
[0055] The second step is ion exchange, in which the halogen ions are exchanged...
Embodiment 3
[0059] Use (PP13)TFSI(PP13 to represent N-methyl-N-propylpiperidinium ion, TFSI to represent N(CF 3 SO 2 ) 2 - ) as an example the first step is quaternization, and the piperidine salt containing halogen is obtained with N-methylpiperidine, and the reaction is as follows:
[0060] Specific operation: 19.8g (0.2mol) N-methylpiperidine was dissolved in 30ml of acetonitrile, the solution was poured into a 250ml three-necked flask, stirred at 40°C under an Ar gas atmosphere, and then slowly dripped into bromopropane 24.6g ( 0.2mol), after reacting for 6 hours, the reaction solution was added to a beaker filled with 150ml ethyl acetate, a large amount of white precipitates were separated out, filtered to obtain precipitates, recrystallized in 20ml of acetonitrile, filtered, and dried in vacuum for 36h to obtain [PP13 ] Br 18g (yield 81%).
[0061] The second step is ion exchange, in which the halogen ions are exchanged for the required negative ions to obtain the ionic li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com