Albumin nanosphere medicine composition and its preparation method and application method
A technology of albumin and nanospheres, which is applied in drug combination, drug delivery, and pharmaceutical formulations, can solve the problems of not paying attention to drug targeting, not considering side effects, and the harm of heavy metal ions and tiny carbon particles. The effects of reducing pain, prolonging the action time, and reducing the possibility of precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
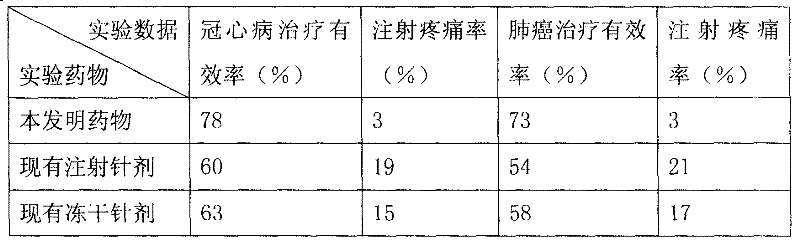
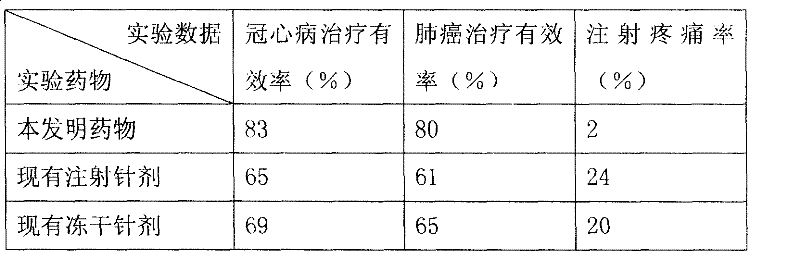
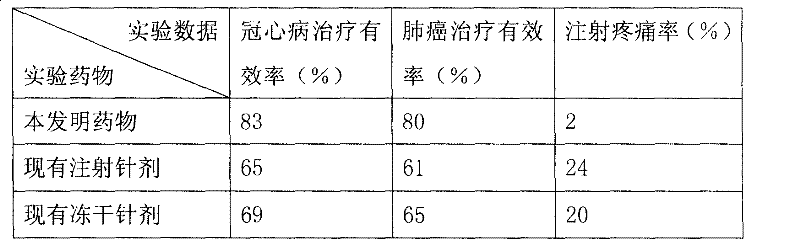
Image
Examples
Embodiment 1
[0046] Preparation of drug-loaded nanospheres for use.
[0047] Formula is as follows (by weight):
[0048] Albumin 1000-2000;
[0049] Tanshinone IIA salt 20-200;
[0050] Vegetable oil 200000-400000;
[0051] PEG6000 100—200;
[0052] N—(2-Mercaptopropionyl)—Glycine 20—100;
[0053] Lysine hydrochloride 20-30.
[0054] 1) Take the amount of albumin dissolved in water to make 200g-300g / L albumin aqueous solution, add the amount of sodium tanshinone IIA sulfonate or / and sodium tanshinone IIA phosphate, dissolve completely, then add the amount of hydrochloric acid Lysine, PEG600 (polyethylene glycol 600), N-(2-mercaptopropionyl)-glycine, completely dissolved;
[0055] 2) Add the solution obtained in step 1) into the amount of vegetable oil, stir rapidly at 100-120°C for 10-20 minutes, and then cool to room temperature;
[0056]3) filter the solution obtained in step 2) with a filter membrane with a pore size of 150nm to obtain nanospheres and filtrate, the average diamet...
Embodiment 2
[0060] Prepare albumin nanosphere glucose injection, formula is as follows (by weight):
[0061] albumin nanosphere combination drug as tanshinone IIA salt 10;
[0062] Hypromellose 20;
[0063] Polygelatin 1250;
[0064] Antioxidant 40;
[0065] Glucose 12500
[0066] Water 250000.
[0067] The antioxidant refers to any combination of reduced glutathione and cystine.
[0068] The specific preparation method is as follows:
[0069] 1) Take albumin nanosphere combination medicine with a particle diameter of 50-150nm and containing the amount of tanshinone IIA salt.
[0070] 2 Add the amount of polygeline, antioxidant and glucose into the amount of water for injection at a temperature of 50-60°C, dissolve completely, adjust the pH value to 5.0, and use an ultrafiltration column with a molecular weight cut-off of 10,000 Daltons Ultrafiltration, adding the amount of hypromellose (treated at 125° C. for 4 hours before adding) to the ultrafiltrate, completely dissolved.
[0...
Embodiment 3
[0079] Prepare albumin nanosphere glucose injection, formula is as follows (by weight):
[0080] Albumin nanosphere combination drug calculated as tanshinone IIA salt 100;
[0081] Hypromellose 10;
[0082] Polygelatin 2500;
[0083] Antioxidant 20;
[0084] Glucose 5000;
[0085] Water 250000.
[0086] The antioxidant refers to one of reduced glutathione, cystine or any combination thereof.
[0087] Mix and dissolve said amount of albumin nanosphere combination drug, hypromellose, polygelatin, antioxidant and glucose in said amount of water, and pack into 1000 sticks.
[0088] The specific preparation method is as follows:
[0089] 4) Take albumin nanosphere combination medicine with a particle diameter of 50-150nm and containing the amount of tanshinone IIA salt.
[0090] 5) Add the amount of polygelatin, antioxidant and glucose into the amount of water for injection at a temperature of 50-60°C, dissolve completely, adjust the pH value to 5.0, and use an ultrafiltrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com