Manufacture of boron-containing compound using cotton balls hydrothermal method and its comprehensive utilization
A technology of soda calcium stone and boron compound, which is applied in the direction of boron oxide compound and borate, and can solve the problem of difficulty in producing pure borate calcium salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
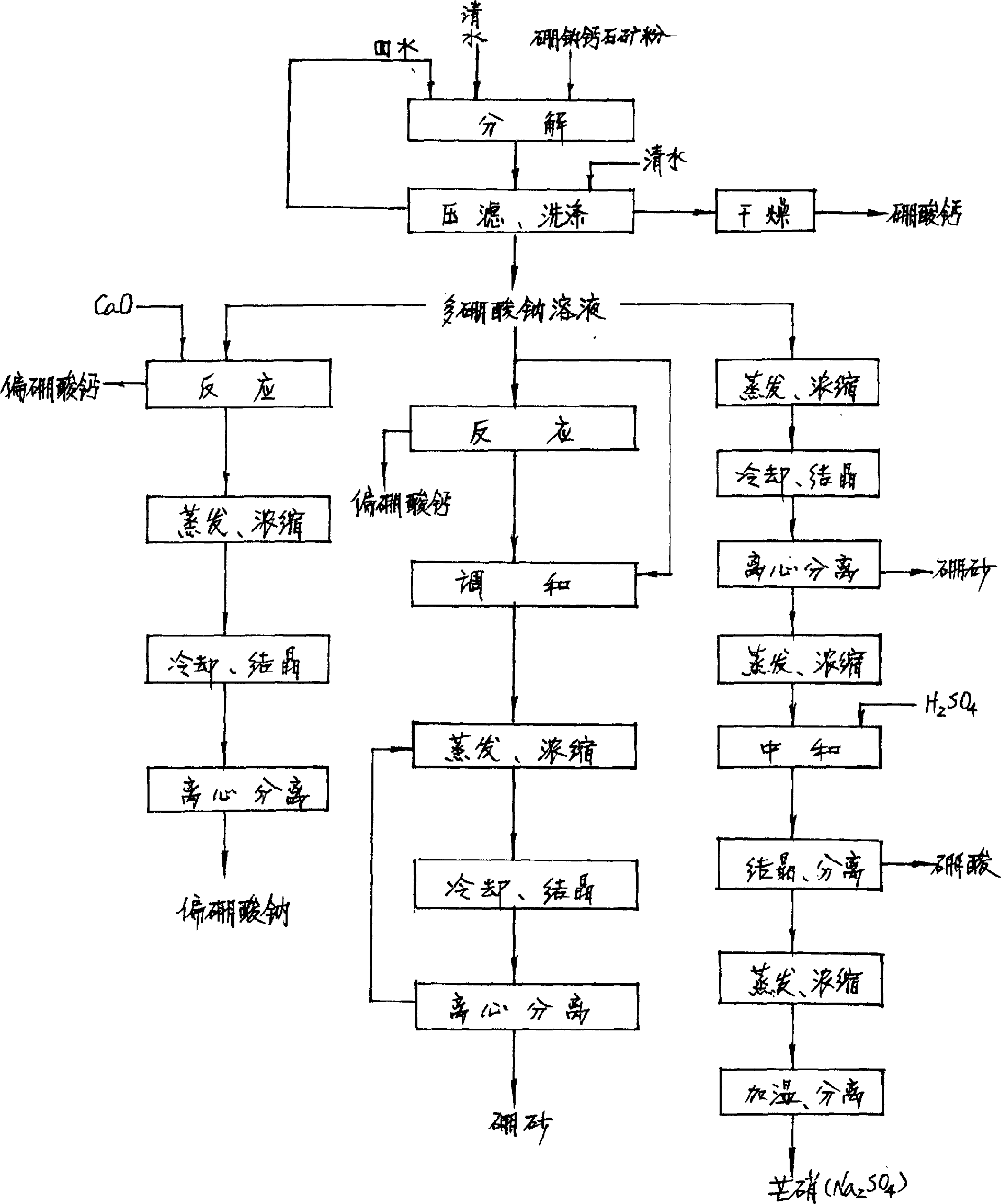
[0043] In an effective volume of 6 meters 3 In a pressure reaction tank equipped with a steam jacket, add 2 meters of boron-calcium washing solution and 2 meters of clear water 3 , start stirring, and add more than 95% of the 180-mesh sodium borate ore powder (containing B 2 o 3 3.8% CaO12.23% Na 2 (06.77%) 1000kg, the pipeline valve of going in and out of the reaction tank is closed, the feeding hole cover is sealed tightly, and steam is heated and boosted. When the pressure in the tank reaches 0.4Mpa, the temperature is 150°C. Keep the pressure and temperature in the tank constant, decompose for 6 hours, then feed the steam of 0.6Mpa to press the material in the tank to the filter press for liquid-solid separation, keep the inlet pressure of the filter press at 0.4Mpa, and the filtrate (polyboric acid Sodium salt solution) is collected in polyborate storage tank, until no filtrate flows out, obtains filtrate 4.03 meters 3 , specific gravity 1.06, component B 2 o 3 45g / ...
Embodiment 2
[0045] In an effective volume of 6M 3 In a pressure reaction tank equipped with a steam heating jacket, add boron-calcium washing solution 2.2M 3 , clean water 2.2M 3 . Start stirring, add more than 95% through 180 mesh clinoborite ore powder (containing B 2 o 3 44.13% CaO 14.7% Na 2 (07.86%) 1000kg, the pipeline valve of going in and out of the reaction tank is closed, the feeding hole cover is sealed tightly, and steam is heated and pressurized. When the pressure in the tank reaches 0.9Mpa, the temperature is 180°C. Keep the pressure and temperature in the tank constant and decompose for 10 hours. Then slowly open the discharge valve, press the material in the tank to the filter press for liquid-solid separation, keep the inlet pressure of the filter press at 0.4Mpa, when the pressure in the tank drops to 0.4Mpa, feed 0.6Mpa steam , continue to press the material in the tank to the filter press until the material in the tank is exhausted and the filter press emits ste...
Embodiment 3
[0047] In a volume of 6M 3 In the reaction tank with stirring, pump into the sodium polyborate solution 4.03m that obtains by example 1 3 , start stirring, drop into 127.7kg of slaked lime powder containing CaO72%, react at room temperature, react for 5 hours, stop the reaction at PH=10.5, pump the slurry to the filter press, keep the pressure before the machine at 0.4Mpa, and the filtrate flows to metaboric acid Sodium dilute liquid storage tank, after the filtrate drips out, use 0.3m 3 The filter cake is washed with water, and the washing liquid also flows into the dilute liquid tank. Finally, dry the filter cake with 0.4Mpa air until no water drops flow out. Unload the filter cake to obtain 395kg containing B 2 o 3 28.95% CaO2 3.28% wet filter cake, the wet filter cake is sent to the drying process, dried with a hollow paddle agitating dryer, keeping the material at 130°C, and staying for 2 hours to obtain B-containing 2 o3 42.46% CaO3 4.07% Na 2 O0.18% calcium metabo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com