The anti-platelet membrane glycoprotein VI monoclonal antibody
A technology of membrane glycoproteins and platelets, applied in the direction of antibodies, anti-animal/human immunoglobulins, immunoglobulins, etc., can solve problems such as difficulty in obtaining high-titer human antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0055] Shown in embodiment 7.
[0056] The antibody of the present invention has the effect of inhibiting human platelet aggregation caused by collagen. Here, the platelet aggregation can be measured by a known method. For example, a platelet aggregation energy measuring device can be used, and the light transmittance is used as an index to calculate the aggregation rate. Usually, the aggregation rate at the point with the maximum light transmittance (hereinafter referred to as called the maximum aggregation rate). In the method described in Example 6 described later, the antibody of the present invention reduces the maximum aggregation rate at a concentration of preferably 10 μg / mL or less, more preferably 1 μg / mL or less, further preferably 0.1 μg / mL or less To preferably 50% or less of the control, more preferably 30% or less of the control, further preferably 20% or less of the control, most preferably 10% or less of the control. The method for measuring inhibition of co...
Embodiment 1
[0127] Example 1 Preparation of Human Soluble GPVI-Fc
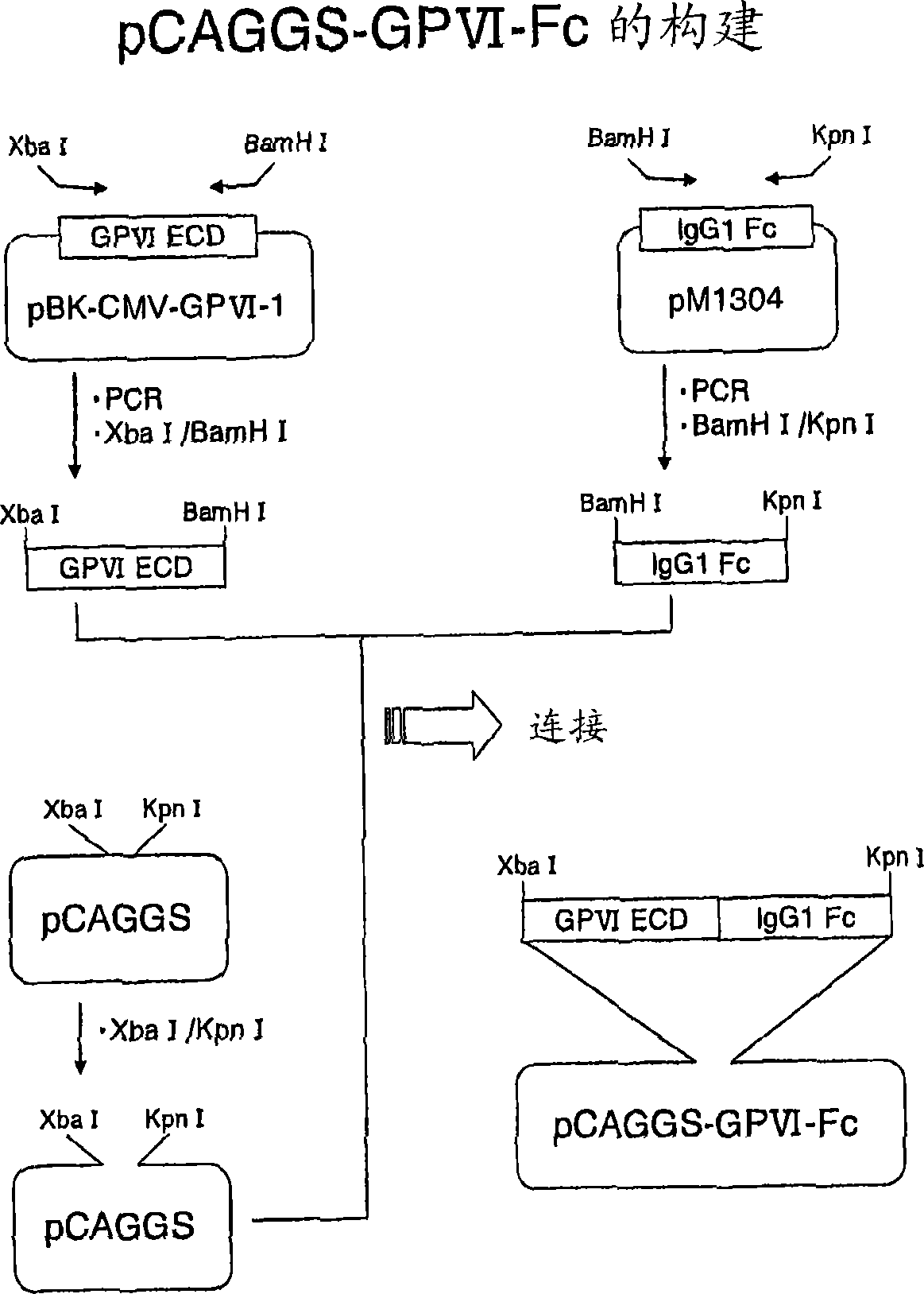
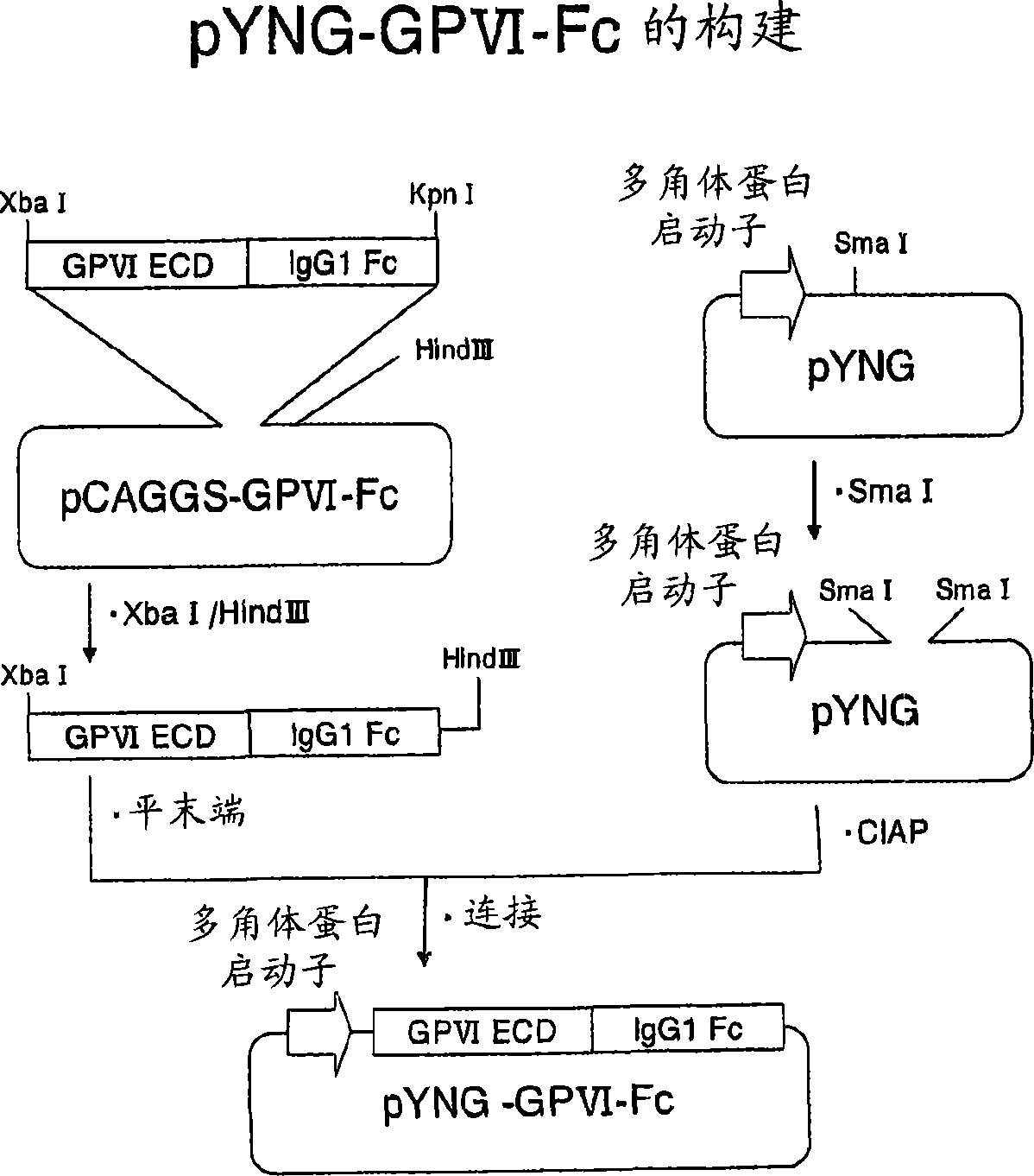
[0128] A fusion protein (GPVI-Fc) of the extracellular domain of human GPVI and the Fc fragment of human IgG (GPVI-Fc) was prepared as an antigen for in vitro immunization and screening. The DNA manipulation was not particularly limited, and was carried out according to the second edition of Molecular Cloning (Molecular Cloning A Laboratory Manual 3rd., Joseph S., et al., Cold Spring Harbor Laboratory Press (2001)).
[0129] The GPVI-Fc expression plasmid was prepared by genetic engineering in the following order. First, using the plasmid pBK-CMV-GPVI-1 (Biochem Biophys. Res. Commun. 2000 August No. 14; 277(1): 27-36) incorporating the human GPVI cDNA cloned by Jiang Jiao et al. Sense primer-1 (SEQ ID NO.33, 5' end side contains restriction enzyme Xba I recognition sequence) and antisense primer-1 (SEQ ID NO.34, 5' end side contains restriction enzyme BamH I recognition sequence) PCR was performed to obtain cDNA encodin...
Embodiment 2
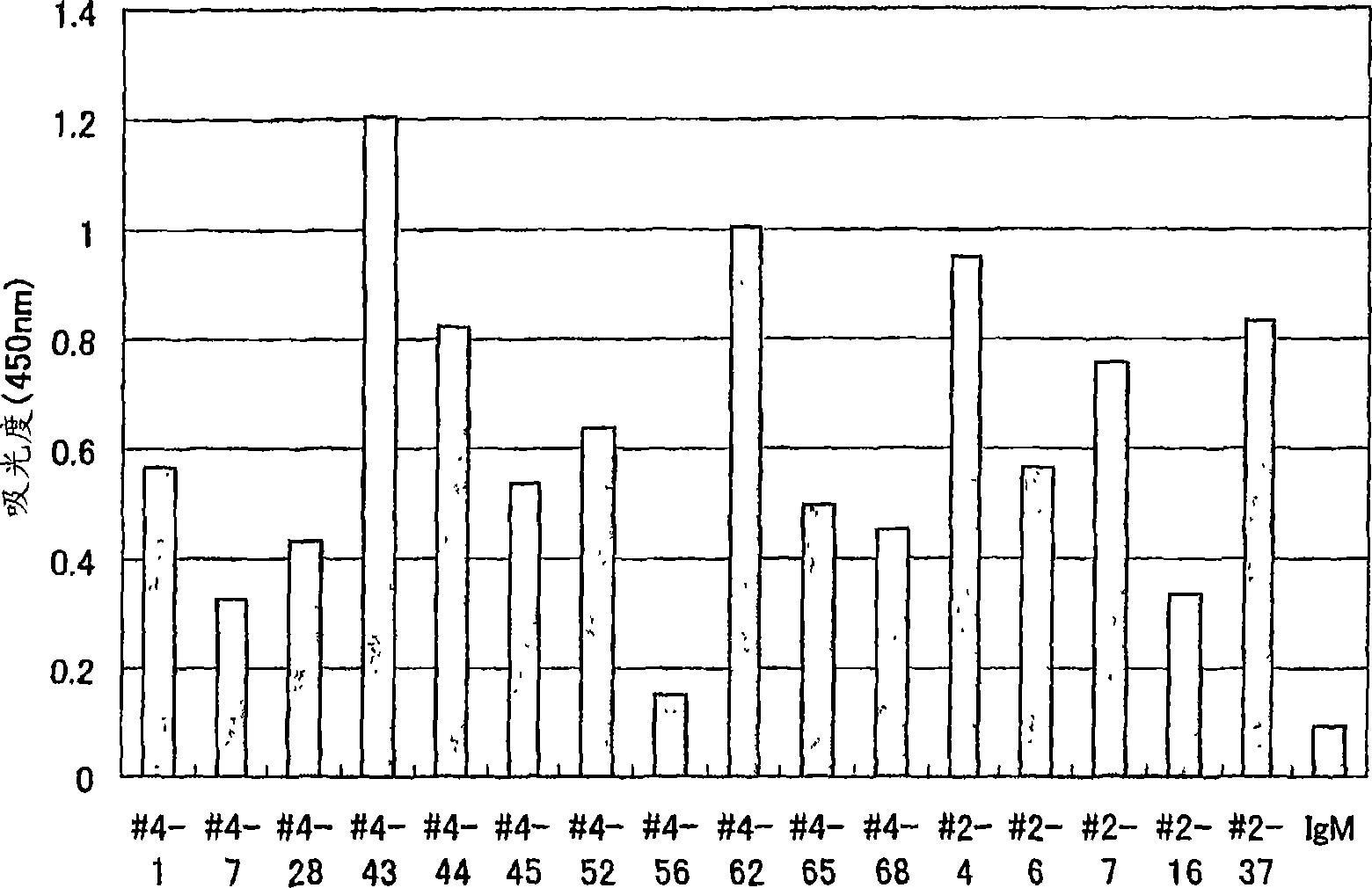
[0132] Example 2 Preparation of anti-GPVI monoclonal human antibody using human peripheral blood lymphocytes with anti-GPVI autoantibodies
[0133] The anti-GPVI monoclonal human antibody was prepared by fusing lymphocytes from blood donors confirmed to have GPVI autoantibodies with myeloma cells as follows. First, 6 ml of heparin-added blood obtained from aseptic blood collection from donors with written consent was placed in a Leucosep centrifugal separation tube (Greiner) added with 3 ml of Ficoll-Plus (Amersham Pharmacia Biotech AB), centrifuged at 1000 g, and lymphocytes were recovered components. The resulting lymphocyte fraction was washed twice with 800g centrifugation in Dulbecco's PBS (hereinafter referred to as D-PBS), then suspended in Hybridoma-SFM (Invitrogen) containing 10% FCS (fetal calf serum) to obtain 7.4 × 10 7 cells.
[0134] Add 2.5 μg / ml PHA-L (Sigma), 20 μg / ml LPS (DIFCO), and 10 μg / ml purified GPVI-Fc described in Example 1 to the above-mentioned ly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com