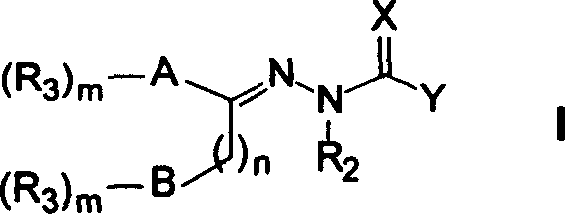
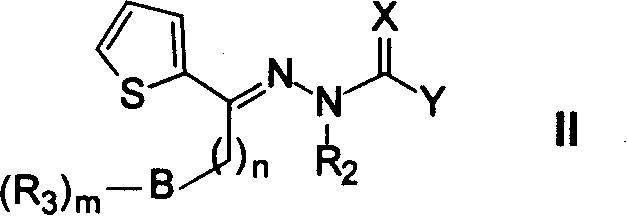
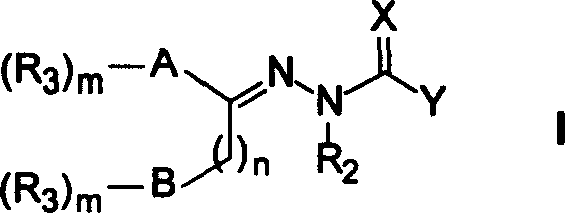
Heteraromatic ring thiosemicarbazone compound, and its derivatives and their use forpreparing antitumour medicine
A technology of thiosemicarbazone and compound, which can be used in antitumor drugs, drug combination, organic chemistry and other directions, and can solve problems such as no antitumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] The synthesis of embodiment 1 2-pyridyl-benzophenone
[0098] A solution of 2-cyanopyridine (104 g, 1 mol) in THF (200 mL) was added dropwise into freshly prepared phenyl Grignard reagent (1.5 L ether-THF solution, 1 mol) at room temperature, and the mixture was stirred overnight. 20% aqueous ammonium chloride (600 mL) was added and stirred for 2 hours, then extracted with ethyl acetate (3 x 500 mL). The extract was dried with anhydrous sodium sulfate, filtered and concentrated to dryness to obtain the product which could be directly used in the next step (Example 4).
Embodiment 2
[0099] Example 2 Synthesis of 2-pyridyl-2-thienyl ketone
[0100] As in Example 1, the newly prepared 2-thienyl Grignard reagent (prepared from 2-bromothiophene by a conventional method) was reacted with 2-cyanopyridine to obtain the product (directly used in Example 5).
Embodiment 3
[0101] Embodiment 3 N, the synthesis of N-dimethyl-N'-thiosemicarbazide
[0102] Carbon disulfide (3.8 g, 50 mmol) was added dropwise to an aqueous solution (50 mL) of sodium hydroxide (2.4 g, 60 mmol) and dimethylamine (5.45 g, 33% in water, 40 mmol) at room temperature middle. After the reaction was stirred for 2 hours, aqueous sodium chloroacetate (5.8 g, 50 mmol) (30 mL) was added dropwise and stirring was continued for 20 hours. The reactant was acidified with dilute HCl solution to pH = 3, and the precipitate was filtered to obtain 4.2 g of the intermediate. The intermediate was mixed with sodium hydroxide (0.9 g, 20 mmol), hydrazine hydrate (2 ml, 85%) and water (12 ml), heated to reflux for 4 hours, and a solid precipitated after cooling. After filtration, the solid was dissolved in hot dichloromethane, and the filter residue was removed by filtration. The filtrate was concentrated to obtain the crude product, which was recrystallized in ethanol to obtain 0.8 g of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com