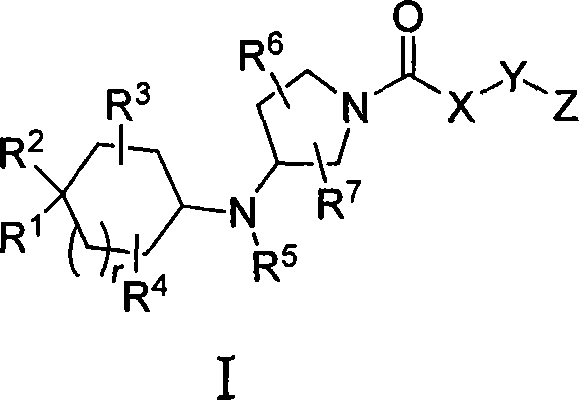
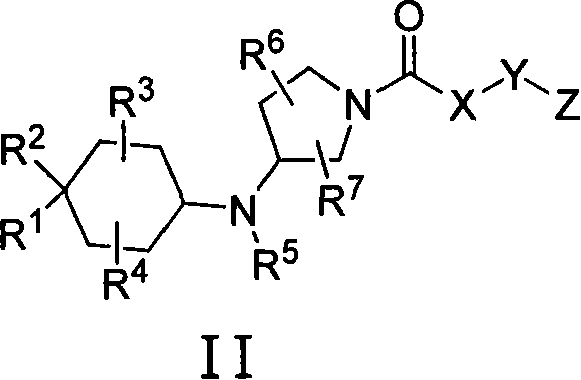
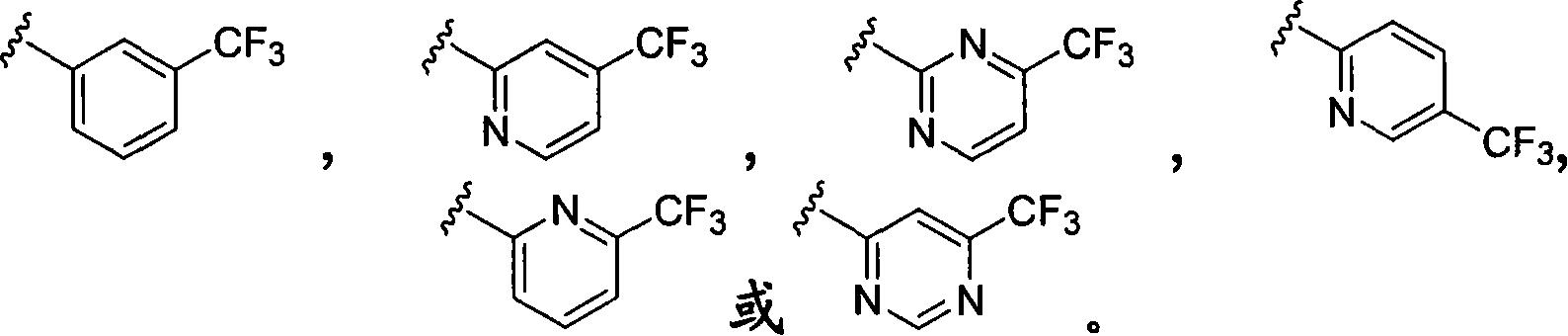
3-cycloalkylaminopyrrolidine derivatives as modulators of chemokine receptors
An alkyl, amino technology, applied in the field of chemokine receptor modulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Step A
[0241]
[0242] (3-Trifluoromethyl-benzamido)acetic acid. At 0°C, to a rapidly stirring solution of glycine (15.014g, 0.20mol) in MeCN (400mL) and 2M NaOH (250mL) was slowly added 3-(trifluoromethyl)-benzoyl chloride (41.714) over 30 min. g, 0.20 mol) in 75 mL of MeCN solution. The turbid yellow solution was stirred at 0°C for 30 min. The reaction mixture was acidified with 3M HCl to pH=3, followed by removal of MeCN on a rotary evaporator. Then the resulting mixture was extracted with EtOAc (400 mL×3). The organic layers were combined, dried, filtered, and concentrated to obtain a pale yellow solid (48.53 g), which was triturated with toluene (500 mL). After filtration, the solid product was washed with cold toluene until the filtrate was colorless. After drying under high vacuum over the weekend, 44.60 g (90%) of a white powder product was obtained. MS(M+H + )=248.1. 1 H NMR(DMSO-d 6 ) δ 12.70 (br s, 1H), 9.17 (m, 1H), 8.20 (dd, 2H), 7.94 (dd, 1H), 7.78 (m, 1H)...
Embodiment 2
[0259] Step A
[0260]
[0261] 8-pyridin-2-yl-1,4-dioxaspiro[4.5]decane-8-ol. To a solution of 2-bromopyridine (14g, 88.6mmol) in anhydrous ether (300mL) cooled at -78°C was slowly added 2.5M n-butyllithium solution (36mL). After the addition, stirring was continued for 1 hour at -78°C. A solution of 1,4-cyclohexanedione mono-ethylene ketal (15 g, 96 mmol) in anhydrous ether (300 mL) was slowly added thereto. When the addition is complete, the mixture is warmed to 0°C and stirring is continued for 1 hour. The reaction was quenched by adding aqueous ammonium chloride (4.5 g) (100 mL). The organic phase was separated, and the aqueous phase was extracted 4 times with methylene chloride. Combine the organic phases and pass MgSO 4 Dry and concentrate. Crystallization from EtOAc gave 7 g of the desired product. The mother liquor was purified on silica gel and eluted with 10% MeOH / EtOAc to give 3 g of the desired product. MS(M+H) + 236.0.
[0262] Step B
[0263]
[0264] 4-hydroxy-...
Embodiment 3
[0269]
[0270] N-(2-{(3S)-3-[(4-Hydroxy-4-pyridin-2-ylcyclohexyl)(methyl)amino]pyrrolidin-1-yl}-2-oxoethyl)- 3-(Trifluoromethyl)benzamide. To N-(2-{(3S)-3-[(4-hydroxy-4-pyridin-2-ylcyclohexyl)amino]pyrrolidin-1-yl}-2-oxoethyl)-3-( Trifluoromethyl)benzamide (49mg, 0.1mmol) and formaldehyde (0.3mL, 37% aqueous solution) in THF (2mL) solution was added Na(OAc) 3 BH (64 mg, 0.3 mmol). After stirring overnight at room temperature, add saturated NaHCO 3 The solution quenches the reaction. The resulting solution was extracted with EtOAc, and the EtOAc layer was dried (MgSO 4 ),concentrate. After preparative HPLC purification, the title compound was obtained as the TFA salt. MS(M+H) + 505.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com