Method for the separation of methyl mercaptan from reaction gas mixtures
A technology of reaction mixture and methyl mercaptan, which is applied in the preparation of organic compounds, mercaptan preparation, chemical instruments and methods, etc., and can solve problems such as unavoidable, difficult to control, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
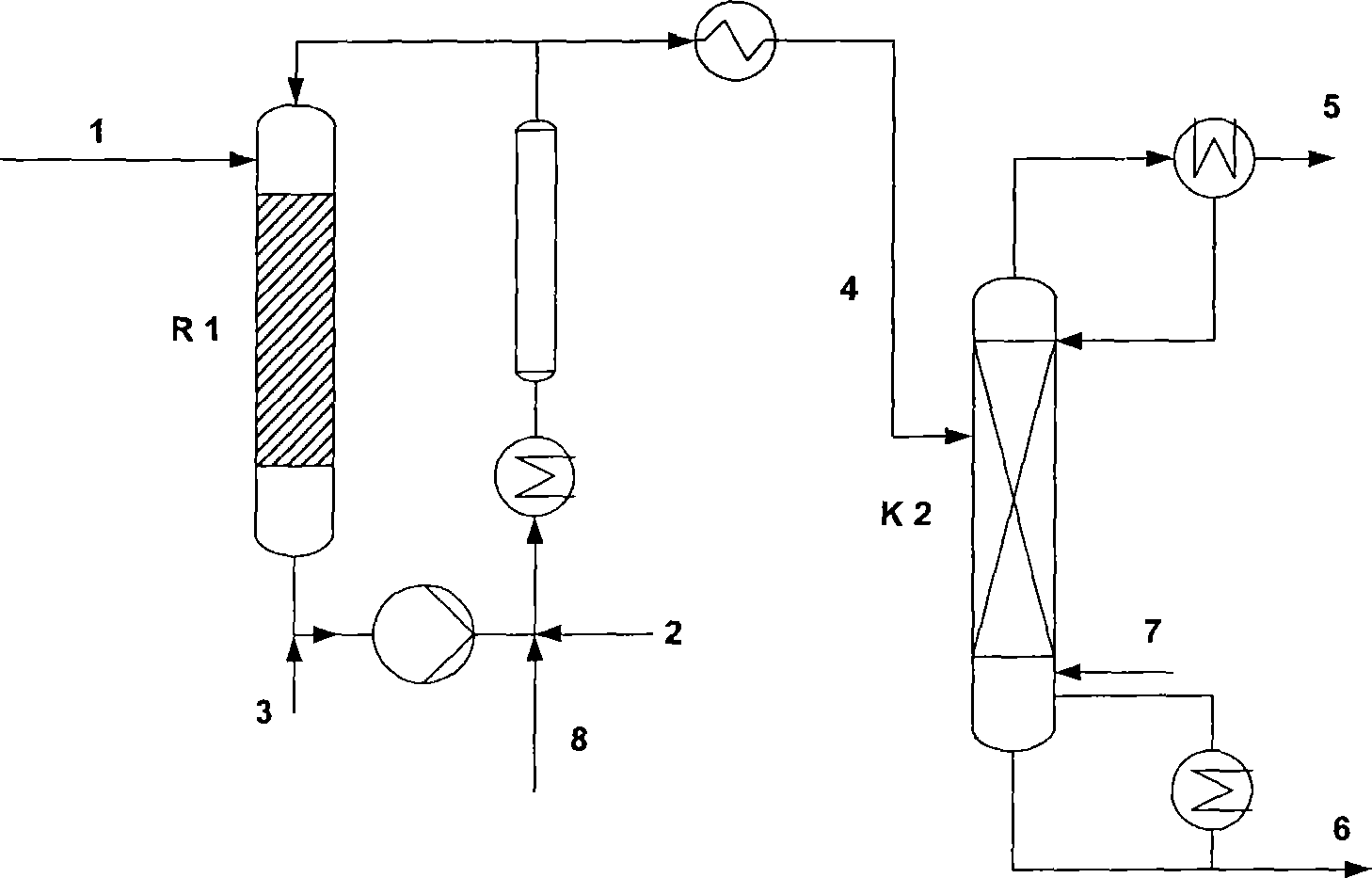
[0025] The present invention passes through attached flowchart ( figure 1 ) to describe as follows:
[0026] Crude methyl mercaptan obtained according to e.g. EP0850923 has the following composition: methyl mercaptan - 93% by weight, dimethyl sulfide - 4.5% by weight, dimethyl ether - 1.5% by weight, water - 1% by weight and traces of methanol or methyl mercaptan Mercaptan ~ 93% by weight, dimethyl sulfide 1.5-5% by weight, dimethyl disulfide 0.2-1% by weight, dimethyl ether 0.5-3% by weight, water ~ 1% by weight and trace methanol, the crude form Mercaptans are fed (in gas and / or liquid form) via 1 or 8 into reactor R1 with MMP, which operates in a cycle at a pressure of 14.5-72.5 psi (1-5 bar) at a temperature as described in DE 2320544 at 50 to 120°C. The acrolein to be converted to MMP is supplied via line 2 and the mixture of various catalysts (such as a mixture of pyridine and acetic acid as described in FR 1520328, or other mixtures of organic bases and organic acids)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com