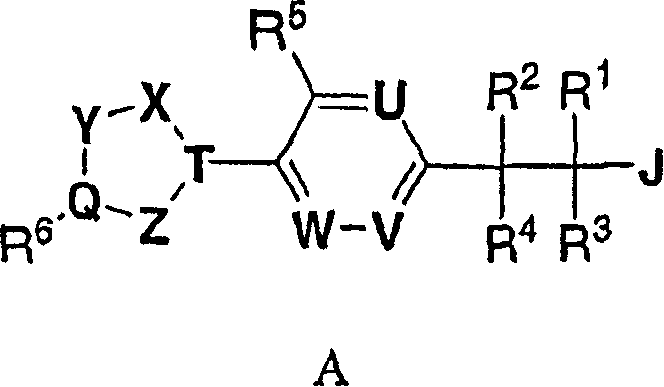
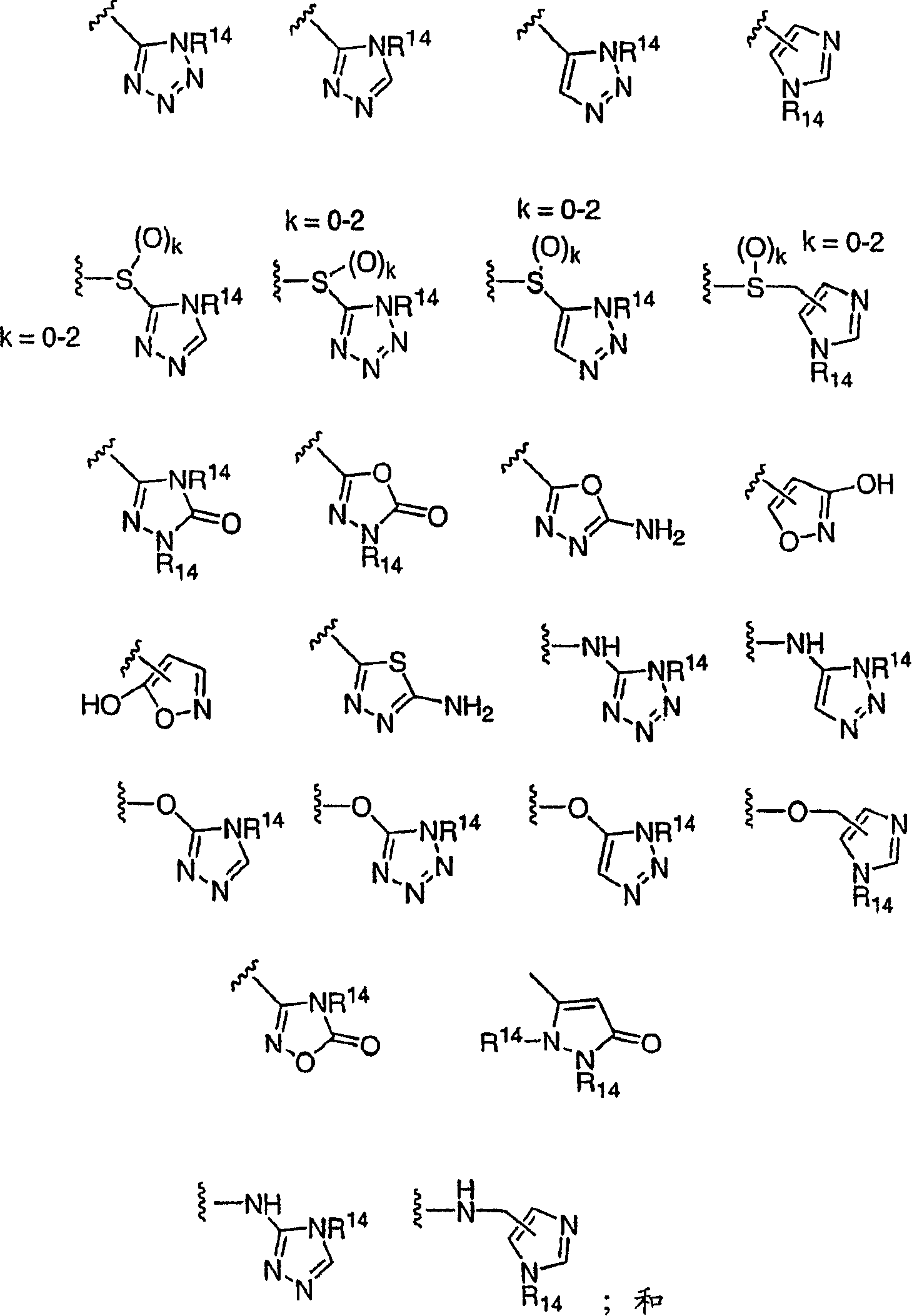
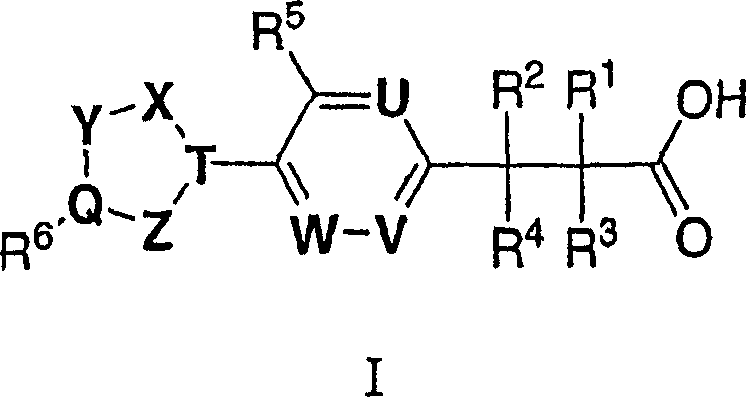
(3,4-disubstituted)propanoic carboxylates as SLP (EDG) receptor agonists
一种取代基、烷基的技术,应用在作为鞘氨醇1-磷酸(内皮分化基因)受体激动剂的(3,4-二取代)丙酸酯领域,能够解决限制用途等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0226] Preparation of N-hydroxyamidine intermediate
[0227] N-hydroxyamidine 1
[0228] N-Hydroxy 3-methyl-4-(2-(tert-butoxycarbonyl)ethyl)benzamidine
[0229] Step A: tert-butyl 3-(3-methyl-4-cyanophenyl)acrylate
[0230] 10.0g (51.0mmol) of 4-bromo-2-methylbenzonitrile in 80ml 1,4-two-alkanes solution with 7.19g (56.1mmol) tert-butyl acrylate, 10.96g (56.1mol) N-methyl di Treat with cyclohexylamine, 228 mg (0.76 mol) 2-(di-tert-butylphosphino)biphenyl and 396 mg (0.38 mol) tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct. The resulting mixture was heated at 70°C for 16 hours and cooled to room temperature. The reaction mixture was filtered through filter paper, and the filtrate was concentrated. The crude product was divided into four fractions. Chromatography (4 Biotage 40M columns, 19:1 v / v Hexane / EtOAc as eluent) followed by pooling of product fractions afforded 10.0 g of the title compound:
[0231] 1 H NMR (500MHz, CDC...
Embodiment 1
[0443] 3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-3-methylphenyl)propanoic acid
[0444] Step A: 3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-3-methylphenyl)propane tert-butyl acid
[0445] 25mg (0.09mmol) N-hydroxyamidine 1, 21mg (0.10mmol) carboxylic acid 1, 26mg (0.14mmol) N-(3-dimethylamino-propyl)-N'-ethylcarbodiimide and 5ml The mixture of acetonitrile was stirred at room temperature for 2 hours and then at 120°C for 16 hours. The reaction mixture was cooled to room temperature and concentrated. Column chromatography on a Biotage 40S (17:3 v / v hexane / EtOAc as eluent) afforded 23 mg of the title compound: 1 H NMR (500MHz, CDCl 3 )δ 1.43(s, 9H), 1.47(d, J=6.2, 6H), 2.57(t, J=7.7, 2H), 2.65(s, 3H), 2.95(t, J=7.7, 2H), 4.76-4.83 (m, 1H), 7.11 (d, J=8.9, 1H), 7.17-7.19 (m, 2H), 7.99 (d, J=8.2, 1H), 8.33 (dd, J=2.3, 9.0, 1H), 8.42 (d, J=2.0, 1H).
[0446] Step B: 3-(4-(5-(3-cyano-4-isopropoxyphenyl)-1,2,4-oxadiazol-3-yl)-3-methylphenyl)prop...
Embodiment 2-7
[0450] The following examples were prepared in a manner similar to that described in Example 1, substituting the appropriate carboxylic acid for carboxylic acid 1 in Step A.
[0451]
[0452]
[0453]
[0454] 1 H NMR (500MHz, CD 3 OD) δ1.40(d, J=5.9, 6H), 2.61(s, 3H), 2.66(t, J=7.8, 2H).2.96
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com