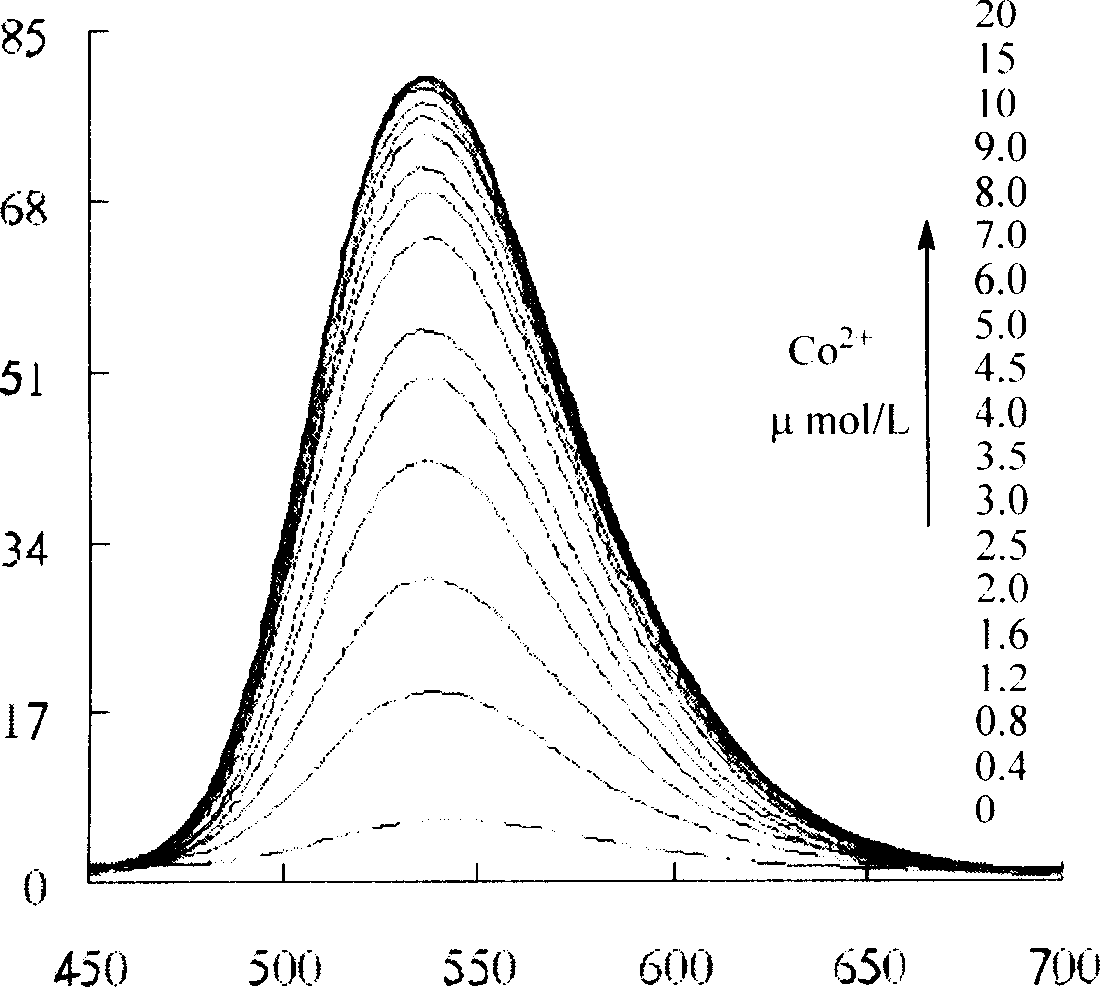
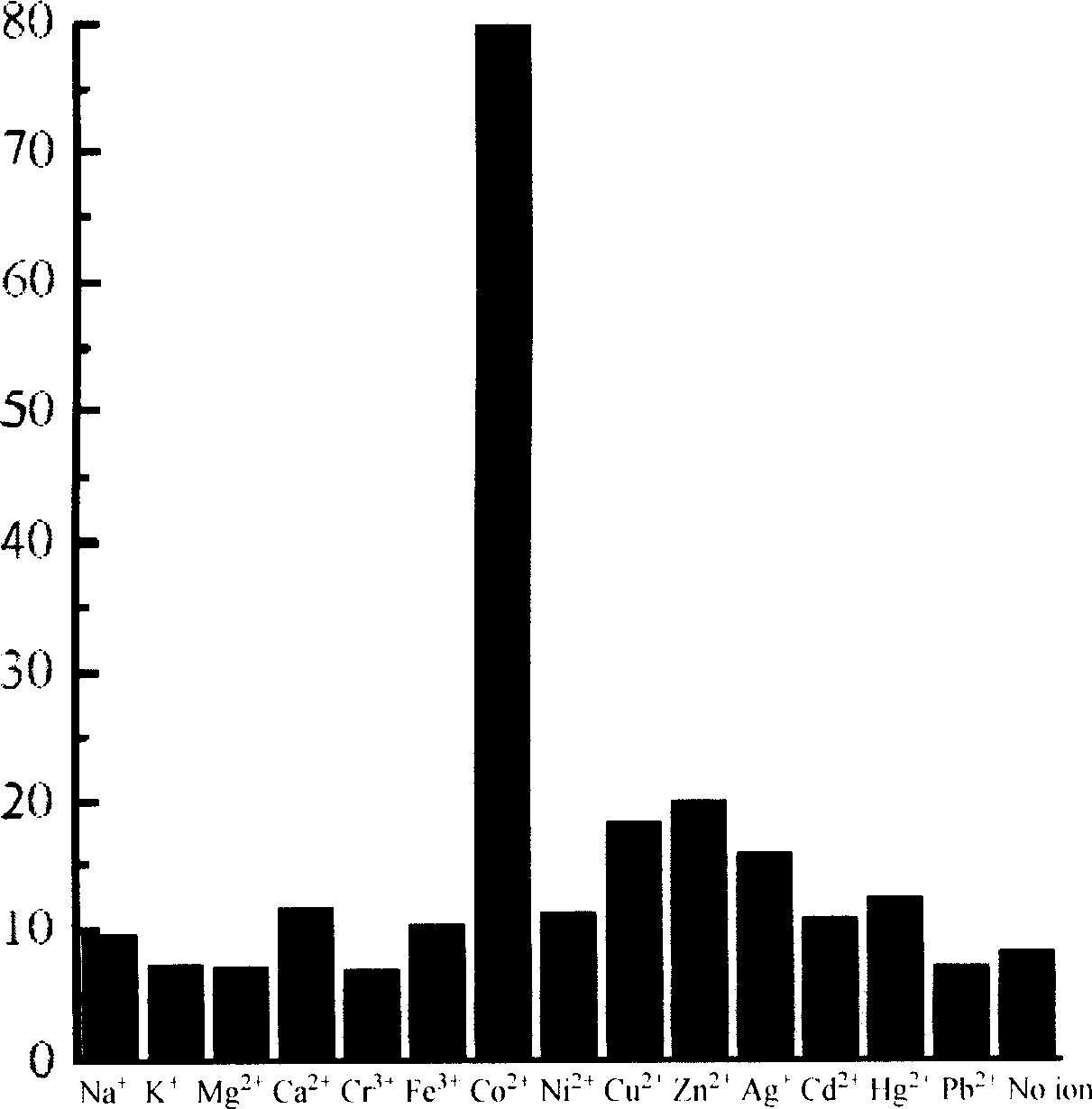
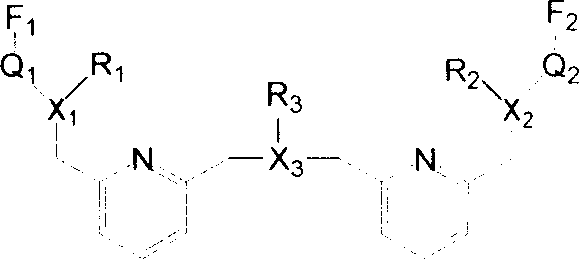
Fluorescent molecular probe and use for inspecting transient metal and heavy metal ion
A fluorescent molecular probe and molecular structure technology, applied in the application field of the fluorescent molecular probe in the identification and detection of transition metal and heavy metal ions, to achieve the effect of good selective fluorescence enhanced recognition and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] N-butyl-4-(2-(2-hydroxyethylamino)ethylamino)-1,8-naphthalimide 180mg (0.385mmol), 2,6-dichloromethylpyridine 70mg (0.40mmol) Dissolve in 50ml of acetonitrile, reflux for about 8h. The solvent was evaporated. The intermediate (P-1-1) was separated by silica gel column chromatography as dark yellow viscous liquid, yield: 69% (130mg).
[0031] The above-mentioned intermediate P-1-1130mg (0.262mmol), monoethanolamine 8mg (0.13mmol) was added to 50ml of acetonitrile solvent, N 2 Protection, heated to reflux for about 7h, cooled to room temperature. After the reaction solution was spin-dried, it was separated by silica gel column chromatography to obtain a dark yellow viscous liquid (P-1). Yield: 55% (70 mg). 1 H-NMR (400MHz, CDCl 3 )δ: 8.46(d, J=7.2Hz, 2H), 8.27(d, J=8.4Hz, 2H), 8.15(d, J=8.2Hz, 2H), 7.44(m, 4H), 6.95(m, 4H), 6.53(br, 2H), 4.16(t, J=7.6Hz, 4H), 3.6-3.8(m, 14H), 3.20(br, 4H), 2.98(br, 6H), 2.75(br, 4H ), 1.70(m, 4H), 1.44(m, 4H), 0.97(t, J=...
Embodiment 2
[0033]
[0034] Add 401 mg (1.09 mmol) of N-hydroxyethoxyethyl-4-piperazinyl-1,8-naphthalimide and 229 mg (1.30 mmol) of 2,6-dichloromethylpyridine to 50 ml of acetonitrile solvent , N 2 Protection, heated to reflux for about 6h, cooled to room temperature. The reaction solution was spin-dried and separated by silica gel column chromatography to obtain a light yellow solid (P-2-1). Melting point: 119.3-120.1°C, yield: 53% (290 mg).
[0035] Add P-2-1203mg (0.398mmol) and monoethanolamine 11mg (0.18mmol) to 30ml of acetonitrile solvent, N 2 Protection, heated to reflux for about 6h, cooled to room temperature. After the reaction solution was spin-dried, it was separated by silica gel column chromatography to obtain a yellow solid (P-2). Melting point: 89.0-90.1°C, yield: 45% (80mg).
[0036] 1 H-NMR (400MHz, CDCl 3 )δ: 8.57(d, J=7.2Hz, 2H), 8.50(d, J=8.4Hz, 2H), 8.40(d, J=8.4Hz, 2H), 7.66(m, 4H), 7.38(d, J=7.2Hz, 2H), 7.30(d, J=10.8Hz, 2H), 7.20(d, J=8.4Hz, 2H), 4.43...
Embodiment 3
[0038]
[0039] Add 380 mg (1.126 mmol) of N-butyl-4-piperazinyl-1,8-naphthalimide and 200 mg (1.136 mmol) of 2,6-dichloromethylpyridine into 50 ml of acetonitrile solvent, N 2 Protection, heated to reflux for about 7h, cooled to room temperature. After the reaction solution was spin-dried, it was separated by silica gel column chromatography to obtain the intermediate as a yellow-green solid (P-3-1). Melting point: 147.0-147.8°C, yield: 58% (290mg).
[0040] Take the above-mentioned intermediate P-3-1172mg (0.360mmol), monoethanolamine 10mg (0.164mmol) and add it to 30ml of acetonitrile solvent, N 2 Protection, heated to reflux for about 6h, cooled to room temperature. After the reaction solution was spin-dried, it was separated by silica gel column chromatography to obtain a golden yellow solid (P-3). Melting point: 85.8-86.9°C, yield: 60% (92mg). 1 H-NMR (400MHz, CDCl 3 )δ: 8.57(d, J=7.2Hz, 2H), 8.50(d, J=8.0Hz, 2H), 8.40(d, J=8.4Hz, 2H), 7.68(t, J=8.0, 2H), 7.63(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com