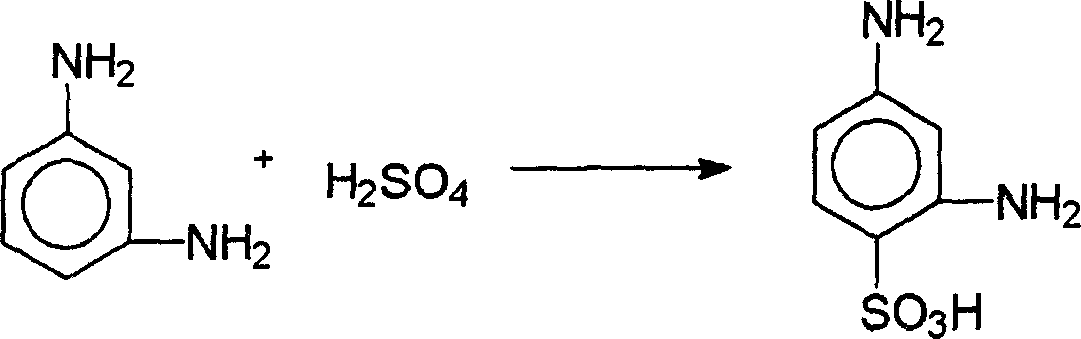Process for synthesizing 2,4-diamino benzene sulfonic acid and its salt
A diaminobenzenesulfonic acid and sulfuric acid technology, applied in 2 fields, can solve problems such as rising production costs and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] In the preparation method of the present invention, the inorganic solvent and the organic solvent may be used alone or in combination. When the inorganic solvent and the organic solvent are used in combination, the specific dosage can be determined according to the above description of the dosage of the inorganic solvent and the organic solvent.
[0028] In the preparation method of the present invention, the sulfonation reaction is carried out in the temperature range of 140-250°C, preferably in the temperature range of 160-210°C, particularly preferably in the temperature range of 170-200°C.
[0029] "Sulfuric acid" as used in the present invention refers to sulfuric acid of any concentration or oleum of any concentration diluted with water to sulfuric acid of any concentration. For example, the sulfuric acid used in the present invention may be 0.1-100% sulfuric acid or any concentration of oleum diluted with water to 0.1-100% sulfuric acid. The sulfuric acid is mor...
Embodiment 1
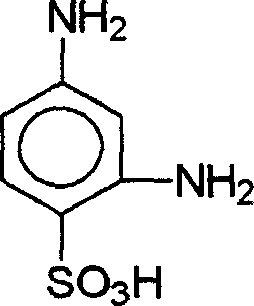
[0038] To a reactor equipped with a condenser with an oil-water liquid separator was added 300 g of o-dichlorobenzene, 100 ml of 98% sulfuric acid (184 g) (equivalent to 180 g of 100% sulfuric acid or 1.84 moles of 100% sulfuric acid) and 100% 100 grams of m-phenylenediamine (equivalent to 0.926 moles), warmed up to 175 ° C, reacted at 175 to 180 ° C for 4 hours, cooled, added 400 ml of water, separated the organic phase and the water phase, the organic phase can be used for the next batch reaction The solvent, the aqueous phase was purified and decolorized to obtain 168.8 g of 2,4-diaminobenzenesulfonic acid. The appearance of the obtained product is white crystal, the chromatographic content is 99% (determined by HPLC), the chemical content is 98% (determined by diazo titration), and the product yield is 95% (calculated by m-phenylenediamine).
Embodiment 2
[0040] To the reactor, add 30 grams of phosphoric acid, 155 grams of 98% sulfuric acid (equivalent to 150 grams of 100% sulfuric acid) and 100 grams of 100% m-phenylenediamine, heat up to 195 ° C, react at 195 to 200 ° C for 6 hours, cool, 400 ml of water was added, and the aqueous phase was purified and decolorized to obtain 168.0 g of 2,4-diaminobenzenesulfonic acid. The appearance of the obtained product is white crystal, the chromatographic content is 99% (determined by HPLC), the chemical content is 98% (determined by diazo titration), and the product yield is 95% (calculated by m-phenylenediamine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com