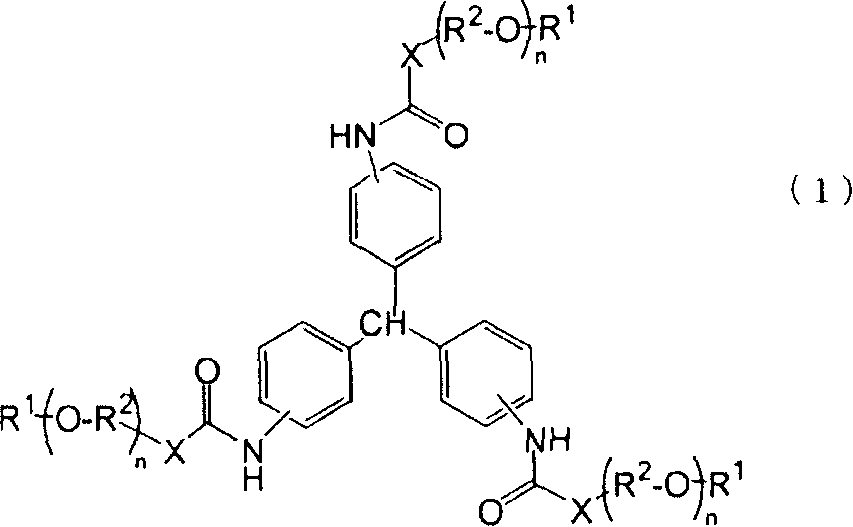
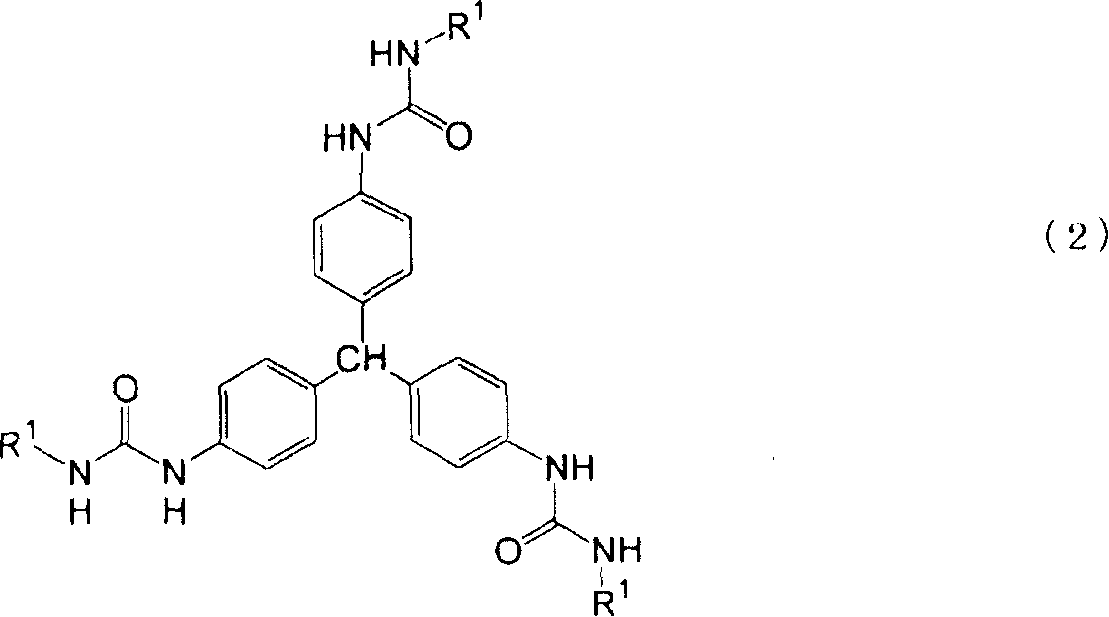
New triphenylmethane derivative, organic gelling agent containing the same, organic gel, and organic fiber
A technology of triphenylmethane and organogel, which is applied in the preparation of urea derivatives, the preparation of organic compounds, organic chemistry, etc., and can solve the problems of inability to express gelling ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A solution of 10 milliliters of anhydrous dichloromethane containing 2.96 grams of octadecylamine (reagents obtained from Kagaku Co., Ltd.) was charged into the reaction vessel, and 10 milliliters of dimethylacetamide (DMAc) was mixed with a dropping funnel. was added to 4.83 g of an ethyl acetate solution containing 27% by weight of triphenylmethane triisocyanate (TPMTI) (“DISMODULE RE” (trade name), available from Sumitomo Bayer Urethane Co. Ltd.; NCO equivalent: 441). The solution was slowly added dropwise to the reaction vessel and stirred at room temperature for 1 hour. By observing the infrared spectrum (KBr method) at 2230 cm -1 The end point of the reaction was determined by the disappearance of the NCO group. The resulting reaction mixture was added to a large amount of distilled water to obtain a precipitate. The resulting precipitate was purified by column chromatography to obtain the target product. As a result, it was confirmed that the amount of the tar...
Embodiment 2
[0065] The same procedure as in Example 1 was repeated except that 4.35 grams of octylamine from Kao Corp. was used instead of octadecylamine. The gel test results are shown in Table 1.
[0066] Elemental analysis value (for C 46 h 70 N 6 o 3 )
[0067] CHN theoretical value (%): 73.2, 9.3, 11.1
[0068] CHN measured value (%): 73.1, 9.4, 11.0
[0069] From the results of infrared analysis, it can be confirmed that the 2331 cm from the isocyanate group (NCO) -1 The absorption peak at disappeared, while the 1636 cm from urea was observed -1 absorption peak at .
[0070] Can determine that gained product is the compound with the chemical structure represented by following formula (8) from the raw material that adds and the result of elemental analysis and infrared analysis:
[0071]
Embodiment 3
[0073] The same procedure as in Example 1 was repeated except that 4.35 g of n-butylamine obtained from Kagaku Co. Ltd. was used instead of octadecylamine. The gel test results are shown in Table 1.
[0074] Elemental analysis value (for C 34 h 46 N 6 o 3 )
[0075] CHN theoretical value (%): 69.6, 7.9, 14.3
[0076] CHN measured value (%): 69.8, 7.8, 14.1
[0077] From the results of infrared analysis, it can be confirmed that the 2331 cm from the isocyanate group (NCO) -1 The absorption peak at disappeared, while the 1637 cm from urea was observed -1 absorption peak at .
[0078]Can determine that gained product is the compound with the chemical structure represented by following formula (9) from the raw material that adds and the result of elemental analysis and infrared analysis:
[0079]
[0080] Organic solvents
Minimum gelling concentration (g / L)
Example 1
Example 2
Example 3
2
×
-
1,1,2,2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com