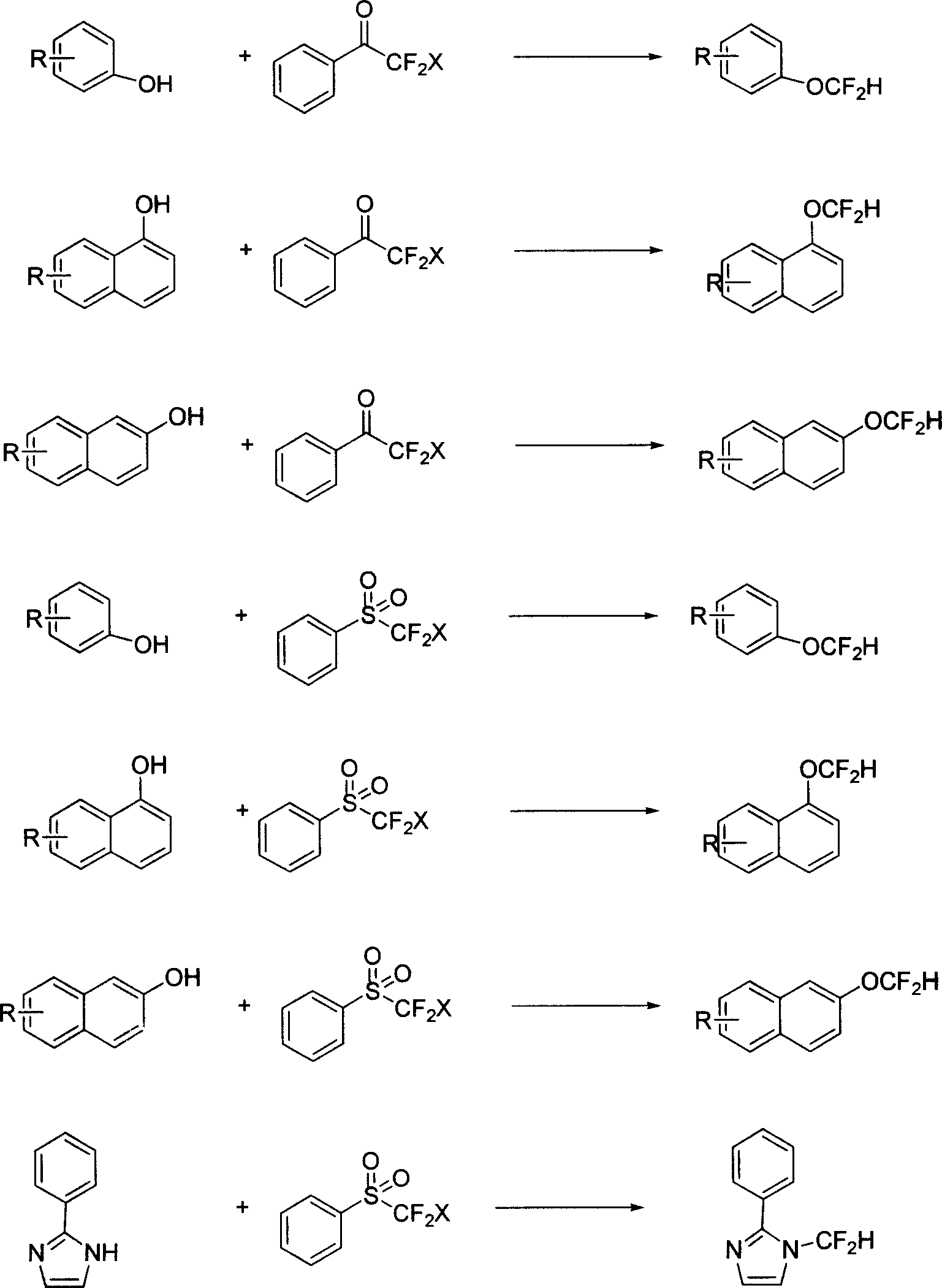
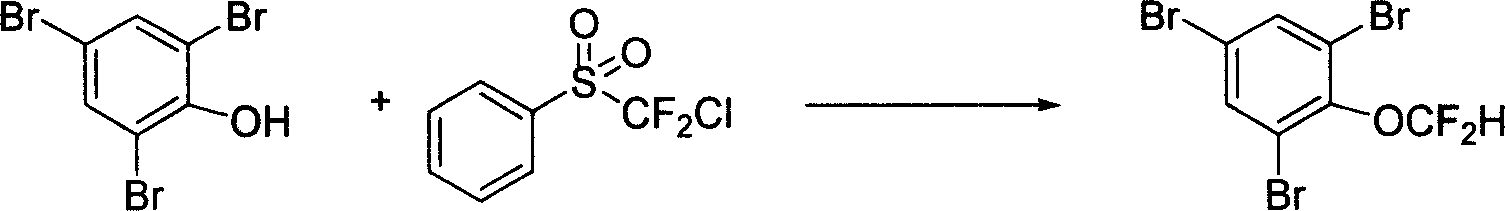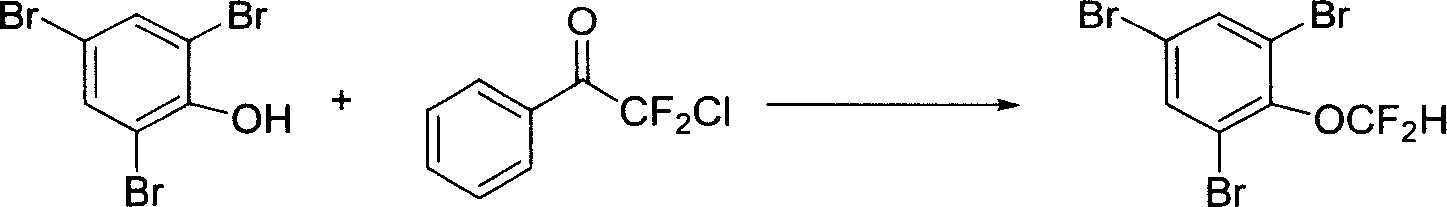Method of synthesizing compound containing difluoromethyl group
A technology of difluoro-halomethyl phenyl ketones and compounds, which is applied in the field of synthesizing compounds containing difluoromethyl groups, can solve the problems of destroying the ozone layer, and achieve the effect of mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] At an oil bath temperature of 50°C, put 0.33 g of 1,3,5-tribromophenol, 5 ml of acetonitrile, 2 ml of 30% KOH aqueous solution, and 0.452 g of difluorochloromethylphenyl sulfone into a sealed tube The reaction was stirred for 4 hours. After the reaction was completed, it was cooled to room temperature, and ethyl acetate and water were added for extraction. The obtained organic phase was washed with saturated brine, then dried by adding anhydrous magnesium sulfate, and the solvent was spin-dried, and 0.32 g of the product 1,3,5-tribromophenyl difluoromethyl ether was separated by silica gel chromatography, 84 %Yield. The reaction formula is as follows:
[0018]
Embodiment 2
[0020] At an oil bath temperature of 80°C, put 0.33 g of 1,3,5-tribromophenol, 5 ml of acetonitrile, 3 ml of 30% KOH aqueous solution, and 0.950 g of difluorochloromethyl phenyl ketone into a sealed tube The reaction was stirred for 4 hours. After the reaction was completed, it was cooled to room temperature, and ethyl acetate and water were added for extraction. The obtained organic phase was washed with saturated brine, then dried by adding anhydrous magnesium sulfate, and the solvent was spin-dried, and the product 1,3,5-tribromophenyl difluoromethyl ether was separated by silica gel chromatography. 0.185 g, 50 %Yield. The reaction formula is as follows:
[0021]
Embodiment 3
[0023] At an oil bath temperature of 50°C, 0.196 g of p-vinylphenylphenol 5 ml of acetonitrile, 2 ml of 30% KOH aqueous solution, and 0.452 g of difluorochloromethylphenyl sulfone were placed in a sealed tube and stirred for 4 hours. get the product 0.199 g, 81% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com