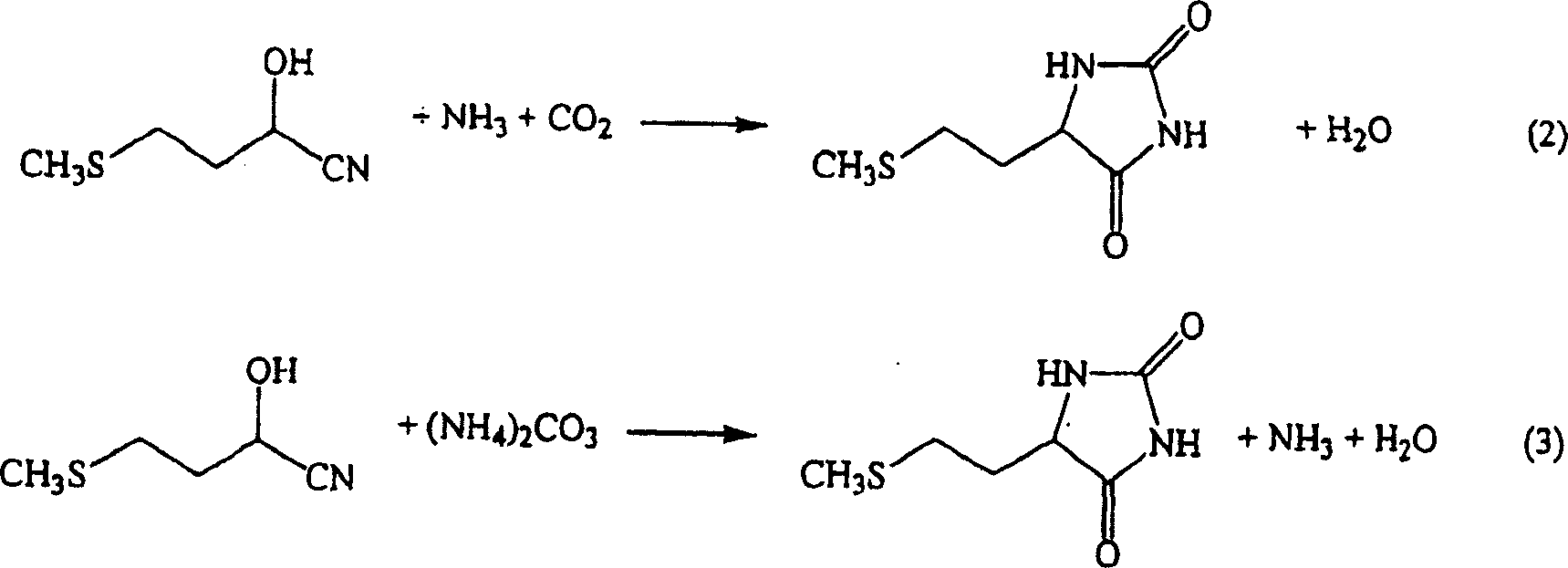
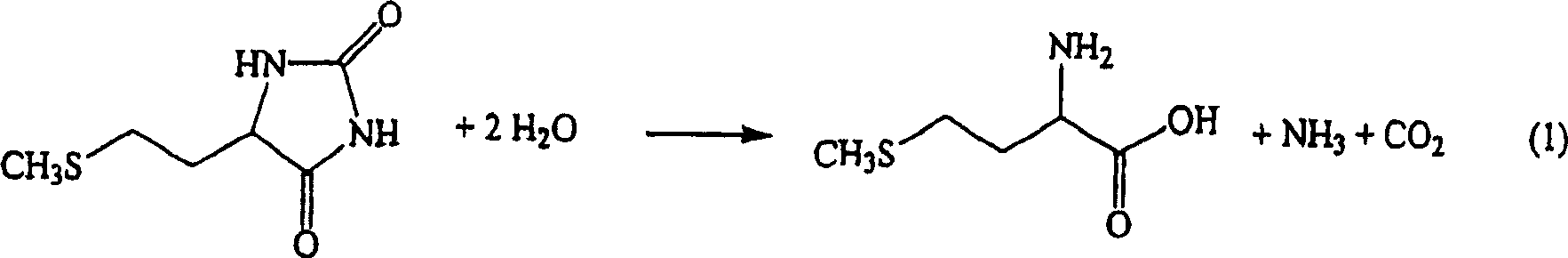Process for producing methionine
A technology of methionine and potassium bicarbonate, applied in the field of preparation of methionine, can solve the problems of loss of methionine and potassium bicarbonate, waste liquid treatment load and high consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [Reaction step (1)]
[0044] By introducing 100 parts / hour into the reactor containing 18.7% of 5-[(2-(methylthio)ethyl)]-2,4-imidazolidinedione aqueous solution, 1.0 parts / hour of potassium hydroxide, 67.6 parts / hour of the primary concentrate of the following first crystal mother liquor, and 25.8 parts / hour of the following second crystal solution, at a temperature of 173-178° C. under a pressure of 0.88 MPa (gauge pressure), with a residence time of 1 hours for the hydrolysis reaction.
[0045] [First crystallization step (2)]
[0046] The reaction solution (133.1 parts / hour) obtained by the previous hydrolysis reaction was mixed with 60.7 parts / hour of water and 0.023 parts / hour of polyvinyl alcohol, and introduced into the crystallization device. Then, crystallization was performed at 20° C. under a pressure of 0.3 MPa (gauge) of carbon dioxide gas to precipitate methionine. The obtained slurry was filtered, and the filtered residue was washed with water and the...
Embodiment 2-4
[0060] The same procedure as in Example 1 was repeated except that the secondary concentration ratio in the third crystallization step (4) of Example 1 was changed to the secondary concentration ratio shown in Table 1.
[0061] The respective recoveries of useful components in the obtained wet cake of the third crystal were measured and shown in Table 1 together with the results of Example 1.
[0062] Example
Embodiment 5-7
[0064] The same procedure as in Example 1 was repeated except that the crystallization temperature in the third crystallization step (4) of Example 1 was changed to the temperature shown in Table 2. The respective recoveries of useful components in the resulting wet cake of the third crystal were measured and shown in Table 2 together with the results of Example 1.
[0065] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com