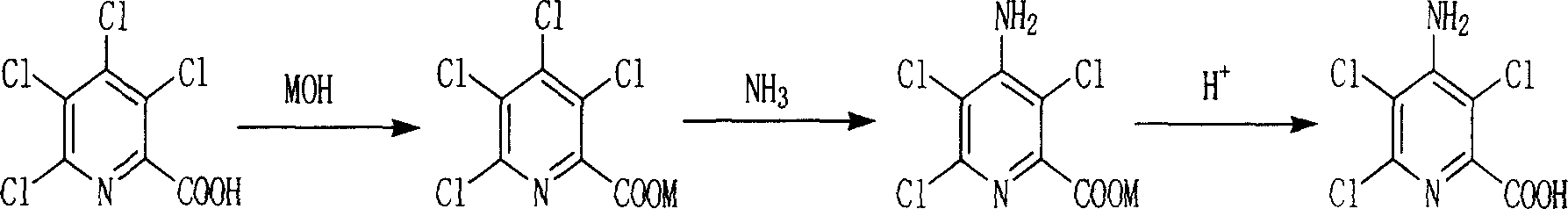
Chemical synthesis method of 4-amino-3,5,6-trichloropyridine-2-formic acid
A technology of tetraclopyridine and triclopyridine, which is applied in the field of synthesis of organic compound herbicides, can solve the problems of high product cost, low product yield, large amount of waste water, etc., and achieve high reaction yield, low product cost, The effect of reaction time reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 1000ml pressure-resistant reactor, add 12.8g (0.3mol) of sodium hydroxide and 750ml water, stir and dissolve, then add 83.1g (0.32mol, 94.2%) of tetraclopyridine, then add 150g liquid ammonia (8.82mol ), under the condition that the temperature is 110-125°C and the reactor is airtight, stir and react for 2.0 hours, release excess ammonia gas after the reaction is completed, then add hydrochloric acid to acidify to pH 1-2, filter and dry to obtain 70.6g product , the yield was 91.3%.
Embodiment 2
[0022] In a 1000ml autoclave, add potassium hydroxide 13.8g (0.3mol) and 750ml water, stir and dissolve, then add 83.1g (0.32mol, 94.2%) of tetraclopyridine, then add 150g liquid ammonia (8.82mol) , under the condition that the temperature is 110-120°C and the reactor is closed, the reaction is stirred for 2.5 hours. After the reaction is completed, excess ammonia gas is released, and then hydrochloric acid is added to acidify to pH 1-2. After filtration and drying, 68.8g of the product is obtained. Yield 89%.
Embodiment 3
[0024] In a 1000ml autoclave, add 750ml of water, then add 83.1g of tetraclopyridine (0.32mol, 94.2%), and then add 320g of ammonium carbonate (3.3mol). Under the conditions, the reaction was stirred for 3.0 hours. After the reaction was completed, hydrochloric acid was added to acidify to pH 1-2, and 67.2 g of the product was obtained after filtration and drying, with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com