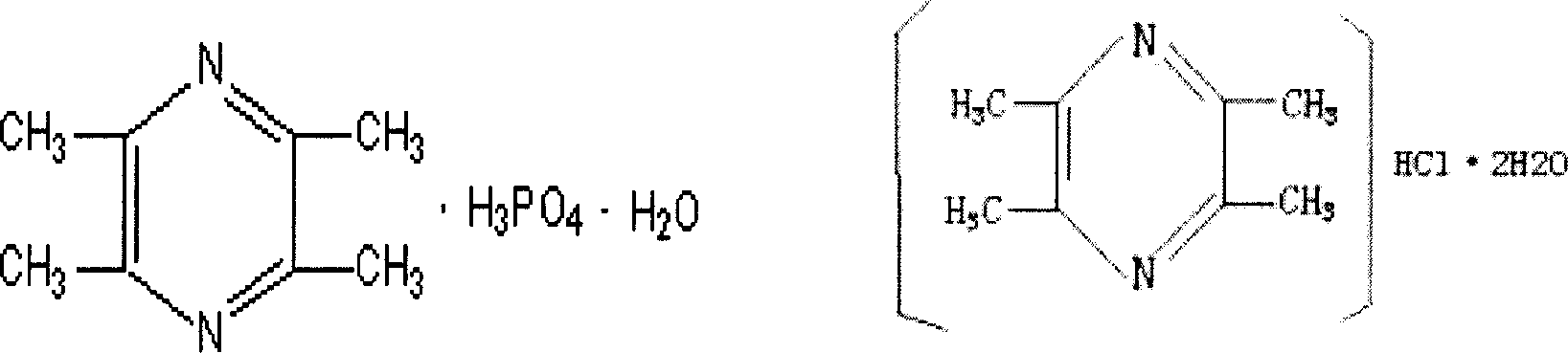
Pharmaceutical composition of ligustrazine and notoginsen triterpenes
A technology of Panax notoginseng saponins and a composition, which is applied in the field of medicine and achieves the effects of wide application prospect, high safety and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of this composition water injection
[0071] prescription:
[0072] Ligustrazine Phosphate 50g
[0073] Panax notoginseng saponins 150g
[0074] Propylene glycol 400ml
[0075] Add water for injection to 5000ml
[0076] A total of 1000 sticks were prepared
[0077] Preparation Process:
[0078]1) Dispose of the pipes and containers used for liquid preparation one day in advance, and rinse them with fresh water for injection before use.
[0079] 2) Ligustrazine phosphate is added to water for injection with a dosing volume of 40% to dissolve completely, and total saponins of Panax notoginseng are added to propylene glycol, heated and stirred to dissolve completely.
[0080] 3) Combine the above solutions and add water for injection to the full amount.
[0081] 4) Add activated carbon for needles with a dosing volume of 0.1%, heat and stir for 15 minutes.
[0082] 5) Decarburization by sand filter stick filtration. Measure and adjust...
Embodiment 2
[0089] The preparation of embodiment 2 this composition powder injection
[0090] prescription:
[0091] Ligustrazine Phosphate 50g
[0092] Panax notoginseng saponins 150g
[0093] Polysorbate 80 100g
[0094] Mannitol 400g
[0095] Add sterile water for injection to 5000ml
[0096] A total of 1000 sticks were prepared
[0097] Preparation Process:
[0098] 1) First, aseptically treat the containers used for liquid preparation, antibiotic glass bottles, rubber stoppers, etc.
[0099] 2) Weigh the raw and auxiliary materials according to the prescription quantity.
[0100] 3) Polysorbate 80 is made into a 20% aqueous solution, the total saponins of Panax notoginseng are heated and stirred to dissolve completely, and ligustrazine phosphate is added into sterile water for injection with a dosing volume of 20% to dissolve completely. Mannitol is added with 40% sterile water for injection, heated and stirred to dissolve completely, the above solutions are combined, and ste...
Embodiment 3
[0108] Embodiment 3 The preparation of this composition sodium chloride injection
[0109] prescription:
[0110] Ligustrazine Phosphate 50g
[0111] Panax notoginseng saponins 150g
[0112] Polysorbate 80 100g
[0114] Add water for injection to 100000ml
[0115] A total of 1000 bottles were prepared
[0116] Preparation Process:
[0117] 1) Dispose of the pipes and containers used for liquid preparation the day before, and rinse them with fresh water for injection before use.
[0118] 2) Polysorbate 80 is prepared into a 20% aqueous solution, the total saponins of Panax notoginseng are added with heating and stirred to dissolve completely, and ligustrazine phosphate is added into water for injection with a dosing volume of 20% to dissolve completely. Dissolve the sodium chloride completely with water for injection with a dosing volume of 40%.
[0119] 3) Combine the above solutions and add water for injection to the full amount.
[0120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com