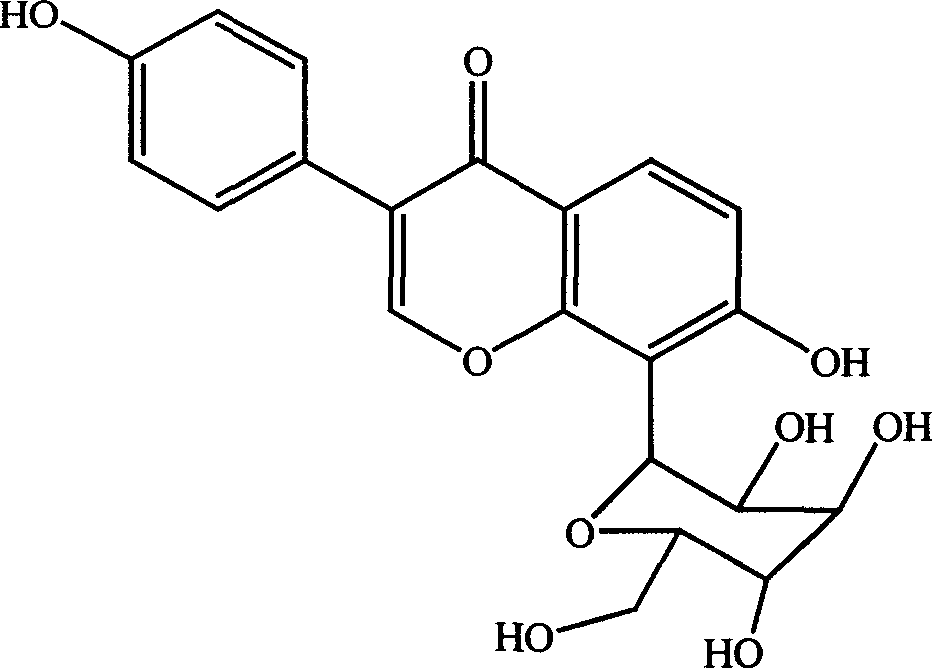
Composition of barrenwort extract and puerarin, preparation process and use thereof
A technology of epimedium extract and composition, applied in the field of medicine, to achieve high safety, prevention of atherosclerosis, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Preparation and identification and content determination of the epimedium extract of embodiment 1 (1)
[0169] Preparation of Epimedium Extract
[0170] Take 1000g of Epimedium, cut into small pieces, add water to decoct 2 times, each time for 1.5 hours, each time add water to 10L. Combine the decoctions, put them in cold storage overnight, filter, concentrate the filtrate to a concentrated solution with a relative density of 1.08 to 1.10, add it to the treated polyamide resin column, wash it with 3 to 4 times the column volume of water, and then wash it with 2 Rinse with 20% ethanol for ~3 times the column volume, discard the washing solution, and then elute with 95% ethanol for 4 to 5 times the column volume, collect the eluate, recover the ethanol, and spray dry to obtain the product.
[0171] Three batches of Epimedium extract were prepared according to the above method, and the yields are shown in Table 8.
[0172] Identification of Epimedium Extract
[0173]Tak...
Embodiment 2
[0185] Preparation and Identification and Content Determination of Example 2 Epimedium Extract (two)
[0186] Preparation of Epimedium Extract
[0187] Take 1000g of Epimedium medicinal material, crush it into medium powder, add 12 times the amount of 70% ethanol, soak for 12 hours, filter, and use the filtrate for later use; add 70% ethanol to heat and extract three times, each time for 1.5 hours, filter, and combine the filtrate , the filtrate was concentrated under reduced pressure to 1000ml, put on the treated polyamide column, first eluted with 15L of distilled water, then eluted with 3L of 15% ethanol, and finally eluted with 20L of 70% ethanol, collected the 70% ethanol eluate, Recover ethanol under reduced pressure and dry it in vacuum to obtain the product.
[0188] Three batches of Epimedium extract were prepared according to the above method, and the yields are shown in Table 9.
[0189] Identification of Epimedium Extract
[0190] According to the identification...
Embodiment 3
[0194] Embodiment 3 The preparation of pharmaceutical composition injection of the present invention
[0195] 1. Prescription:
[0196] Prescription 1
[0197] Epimedium Extract 200g
[0198] Puerarin 200g
[0199] Propylene glycol 700ml
[0200] Add water for injection to 5000ml
[0201] A total of 1000 sticks were prepared
[0202] Prescription 2
[0203] Epimedium Extract 100g
[0204] Puerarin 200g
[0205] Propylene glycol 700ml
[0206] Add water for injection to 5000ml
[0207] A total of 1000 sticks were prepared
[0208] Prescription 3
[0209] Epimedium Extract 200g
[0210] Puerarin 40g
[0211] Propylene glycol 700ml
[0212] Add water for injection to 5000ml
[0213] A total of 1000 sticks were prepared
[0214] Prescription 4
[0215] Epimedium Extract 200g
[0216] Puerarin 20g
[0217] Propylene glycol 700ml
[0218] Add water for injection to 5000ml
[0219] A total of 1000 sticks were prepared
[0220] Prescription 5
[0221] Epimedium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com