Synthesis method of biapenem
A synthesis method and compound technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of complicated operation, low yield, unstable solution, etc., and achieve the effect of high product purity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
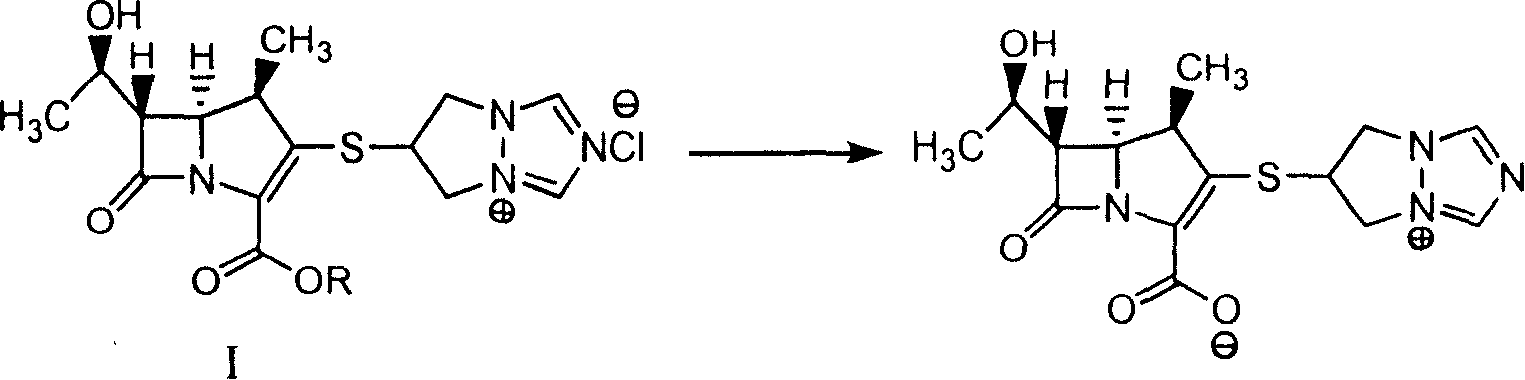
[0021] 30g (63mmol) of 6-[(4R, 5R, 6S)-2-(benzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1 -Azabicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][1,2,4 ] Triazol-4-ium chloride, add 150ml tert-butanol, 50ml ethyl acetate, 450ml 0.30mol / L magnesium chloride buffer solution (pH=5.5), stirring and dissolving, the weight content of adding 10g palladium is 10% palladium Carbon, controlled hydrogenation pressure 5Kg / m 2 , react at 25°C for 3h, filter, wash the aqueous phase with ethyl acetate 300ml×2, add acetone 500ml, stir at -10°C for 2h, filter, wash the solid with acetone 30ml×2, and dry under reduced pressure to obtain parapenem 16.5 g, (yield: 75%, purity: 97.0%).
[0022] In this embodiment, R in the compound of general formula I is PhCH 2 .
Embodiment 2
[0024] 30g (63mmol) of 6-[(4R, 5R, 6S)-2-(benzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1 -Azabicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][1,2,4 ] triazol-4-ium chloride, add 150ml THF, 450ml0.30mol / L sodium acetate damping fluid (pH=5.5), stirring and dissolving, adding the weight content of 1.5g palladium hydroxide is 20% palladium hydroxide carbon , control hydrogenation pressure 10Kg / m 2 , react at 25°C for 2.5h, filter, wash the aqueous phase with ethyl acetate 300ml×2, add acetone 500ml, stir at -10°C for 2h, filter, wash the solid with acetone 30ml×2, and dry under reduced pressure to obtain baper Nan 15.4g, (yield: 70%, purity: 97.5%).
[0025] In this embodiment, R in the compound of general formula I is PhCH 2 .
Embodiment 3
[0027] 30g (57.5mmol) of 6-[(4R, 5R, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7 -Oxo-1-azabicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][ 1,2,4] triazol-4-ium chloride, add isopropanol 150ml, 0.35mol / L zinc acetate buffer salt (pH=6.0) 450ml, stir to dissolve, add platinum carbon (10%) 5g, Control hydrogenation pressure 3Kg / m 2 , reacted at 0°C for 4.0h, filtered, added 400ml of acetone to the filtrate, stirred at -10°C for 1h, precipitated crystals, filtered, washed the solid with acetone 30ml×2, dried under reduced pressure to obtain 12g of parapenem, (yield : 60%, purity: 98.5%).
[0028] In this embodiment, R in the compound of general formula I is 4-O 2 NPhCH 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com