Bisamide bissulfosalt double surface active agent, and its synthesizing method
A surfactant and gemini surface technology, applied in the field of new gemini surfactants and their synthesis, can solve the problems of late Gemini surfactants and limited types of Gemini surfactants, and achieve a simple synthesis method, excellent surface activity, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of TM-10:
[0026] (1) Synthesis of intermediate product N, N'-diethylsulfonic acid (sodium) ethylenediamine
[0027] Add ethylenediamine (0.06mol) and a small amount of water into a 250mL three-neck flask equipped with a stirrer, a thermometer, and a reflux condenser, and slowly add a solution of 2-bromoethylsulfonate (0.1mol) dropwise, at 50-70 ℃ in a constant temperature water bath for 6-8 hours. Then adding absolute ethanol, solids were precipitated, filtered by suction, and dried at 50° C. to obtain 18.84 g of white solids (the yield was about 59%).
[0028] (2) Synthesis of TM-10
[0029] Add 8.921g (0.0276mol) of the intermediate to a 500mL three-neck flask, then add 200mL of distilled water / acetone mixed solution (v:v=1:1) to dissolve it, and slowly add lauroyl chloride dropwise under strong stirring 15.097g (0.0690mol), while adding dropwise triethylamine / acetone solution, keeping the pH value at about 8, and reacting at room temperatur...
Embodiment 2
[0030] Example 2 Preparation of TM-8
[0031] (1) The intermediate product N, N'-diethylsulfonic acid (sodium) ethylenediamine was synthesized according to the method in Example 1.
[0032] (2) Synthesis of TM-8
[0033] Add 5.425g (0.0169mol) of the intermediate to a 500mL three-neck flask, then add 200mL of distilled water / acetone mixed solution (v:v=1:1) to dissolve it, and slowly add decanoyl chloride dropwise under strong stirring 8.048g (0.0423mol), while adding triethylamine / acetone solution dropwise, keeping the pH value at about 8, and reacting at room temperature for 5-8 hours. The reaction solution was cooled in the refrigerator overnight, and precipitates were precipitated, filtered with suction, washed with absolute ethanol three times, and dried at 40°C to obtain a white solid powder, namely TM-8, weighing 3.352g (yield: 29%).
Embodiment 3
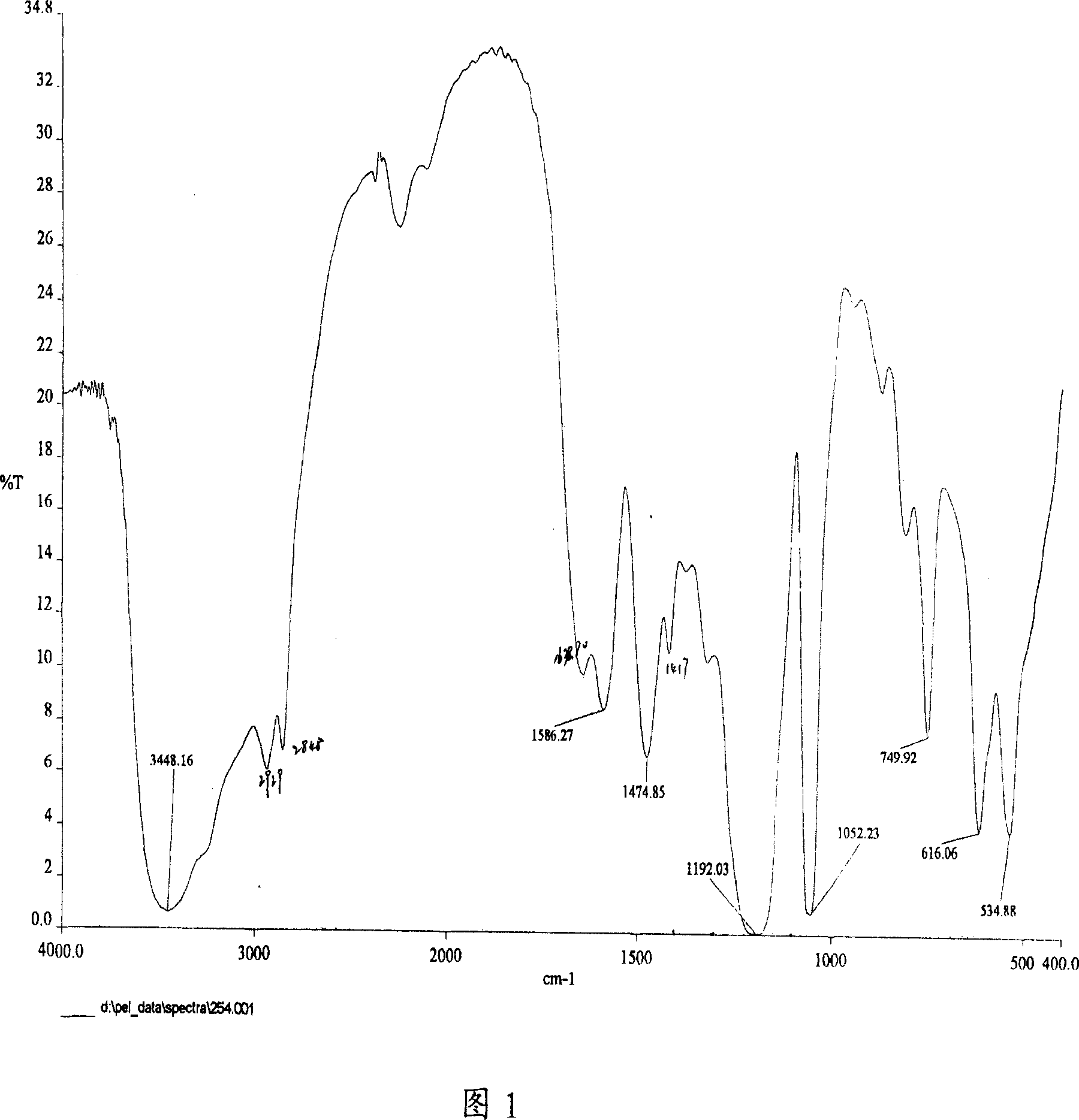
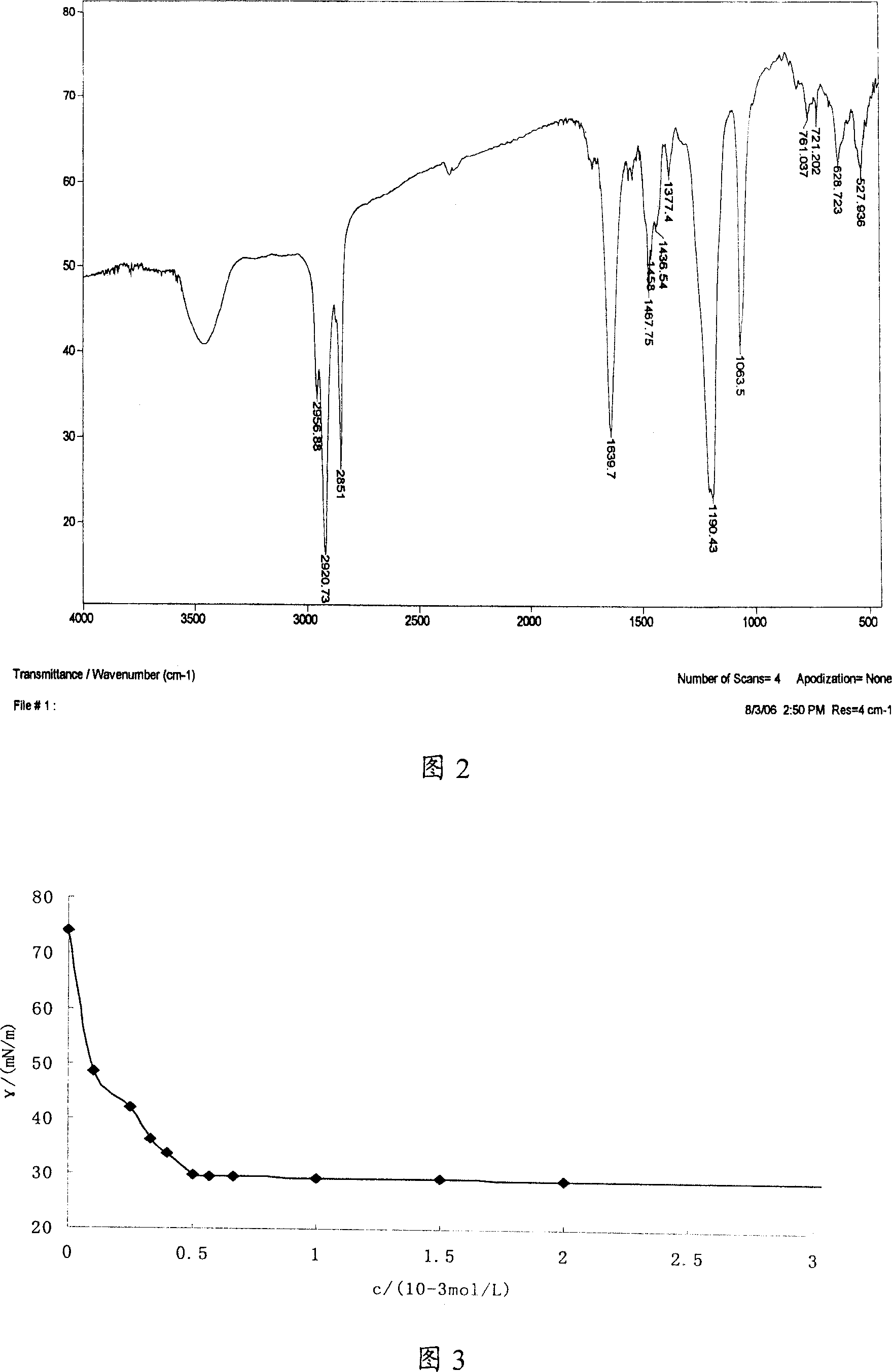
[0034] The surface activity measurement of embodiment 3 TM series surfactants
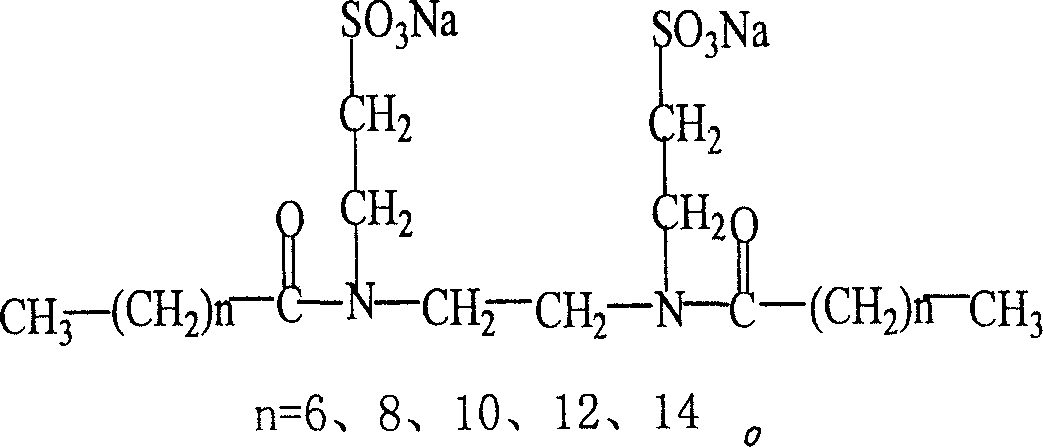
[0035] Surface tension is an important property of liquid. The ability of surfactant to reduce the surface tension of water is an important parameter to evaluate its surface activity. The surface tension of the product solution at different concentrations was measured by the ring method, and the TM-10 aqueous solution was prepared. The surface tension change curve with concentration is shown in Figure 3. The critical micelle concentration value (cmc) and the surface tension (γ cmc ). Experiments have found that the critical micelle concentration of TM series surfactants and the surface tension at critical micelle concentration are low, such as the critical micelle concentration of TM-10 is 5.0×10 -4 mol / L, the surface tension at the critical micelle concentration (γ cmc ) is 29.7mN / m.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com