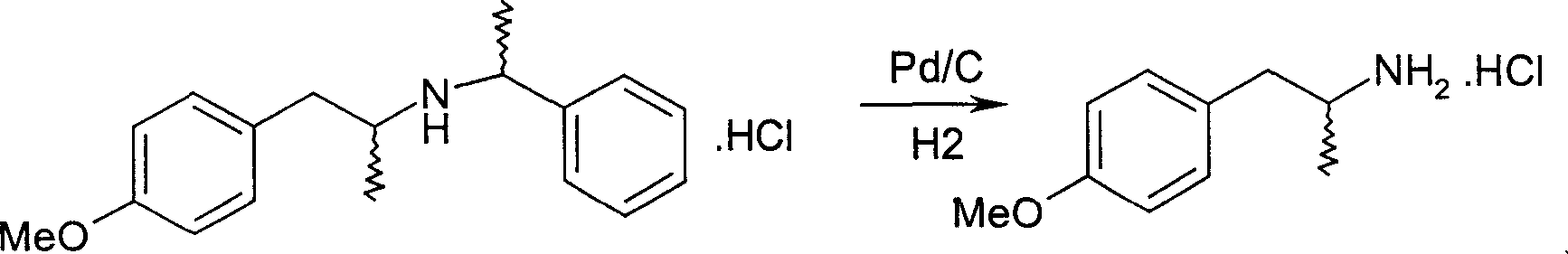
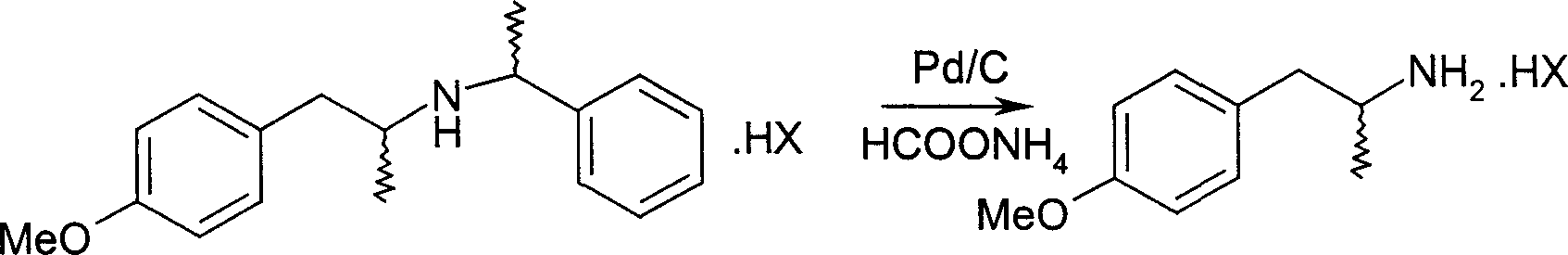
Pharmaceutical synthesis of 4-methoxy-alpha-methyl-phenethylamine
A synthetic method and methoxy technology, applied in chemical instruments and methods, preparation of amino hydroxyl compounds, organic chemistry, etc., can solve the problems of low yield, high equipment requirements, long reaction time, etc., and achieve high reaction yield, The effect of good product purity and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (R, R)-4-methoxy-α-methyl-N-(1-phenyl)-phenethylamine hydrochloride 30g and ammonium formate 12.4g and 10% palladium carbon hydrogenation catalyst 3g, Add 500ml of methanol, heat to reflux for 3 hours, filter the catalyst after cooling, collect the target product R-4-methoxy-α-methyl-phenethylamine hydrochloride 18.4g after recovering the solvent, the yield is 93%. 1 HNMR (CDCl 3 )δ: 1.29(d,3H,-CH 3 ), 2.58~2.75 (m, 2H, -CH 2- ), 3.16 (m, 1H, -CH-), 3.79 (S, 3H, -OCH 3 ), 6.84 (d, 2H), 7.14 (d, 2H); EI-MS (m / z): 166 [(M+H), 100], 149 (48); elemental analysis (C 10 h 16 ClNO) measured value (calculated value, %): C59.46 (59.55), H7.72 (7.80), N6.84 (6.95); [α] D 25 =-22.5°C (C=2, H 2 O); mp: 250-251°C.
Embodiment 2
[0031] (S, S)-4-methoxy-α-methyl-N-(1-phenyl)-phenethylamine hydrobromide 30g and ammonium formate 27.1g and 10% palladium carbon hydrogenation catalyst 1.5 g, add 500ml of ethanol, heat and reflux for 4 hours, filter the catalyst after cooling, collect the target product S-4-methoxy-α-methyl-phenylethylamine hydrobromide 19.2g after recovering the solvent, the yield is 91% . Elemental analysis (C 10 h 16 BrNO) measured value (calculated value, %): C48.65 (48.79), H6.52 (6.55), N5.64 (5.69).
Embodiment 3
[0033] (R, S)-4-methoxy-α-methyl-N-(1-phenyl)-phenethylamine hydrochloride 3g and ammonium formate 6.2g and 5% palladium carbon hydrogenation catalyst 0.6g , add 50ml tetrahydrofuran, heat to reflux for 3 hours, filter the catalyst after cooling, collect the target product R-4-methoxy-α-methyl-phenethylamine hydrochloride 1.78g after recovering the solvent, the yield is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com