Pharmaceutical composition for treatment of immunological disorders
An immunological disease and composition technology, applied in the living field of cells, can solve the problems of difficult to achieve curative effect, inability to effectively suppress immune response, and limited application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: Preparation of the DNA construct according to the coding Ig fusion protein of the present invention
[0078] A. Preparation of a DNA construct encoding the simple fusion monomeric protein LAG3 / Fc
[0079] a. A DNA fragment encoding the soluble extracellular domain of LAG3
[0080] The DNA fragment encoding the soluble extracellular domain of LAG3 can be constructed by PCR, utilizing the sequence (nucleosides whose sequence ID number is 7) with an EcoRI restriction site and a coding leader sequence (1-22 amino acid sequence whose sequence ID number is 8) Acid sequence) primers (nucleotide sequence whose sequence ID number is 1); Nucleotide sequence) antisense primer (nucleotide sequence whose sequence ID number is 4). The template cDNA for this reaction was constructed by reverse transcription PCR (RT-PCR) of mRNA extracted from healthy adult monocytes (T lymphocytes).
[0081] After drawing blood from healthy adults, it was diluted 1:1 with RPMI-1640 (G...
Embodiment 2
[0100] Embodiment 2: Preparation of the DNA construct according to the coding Ig fusion protein of the present invention
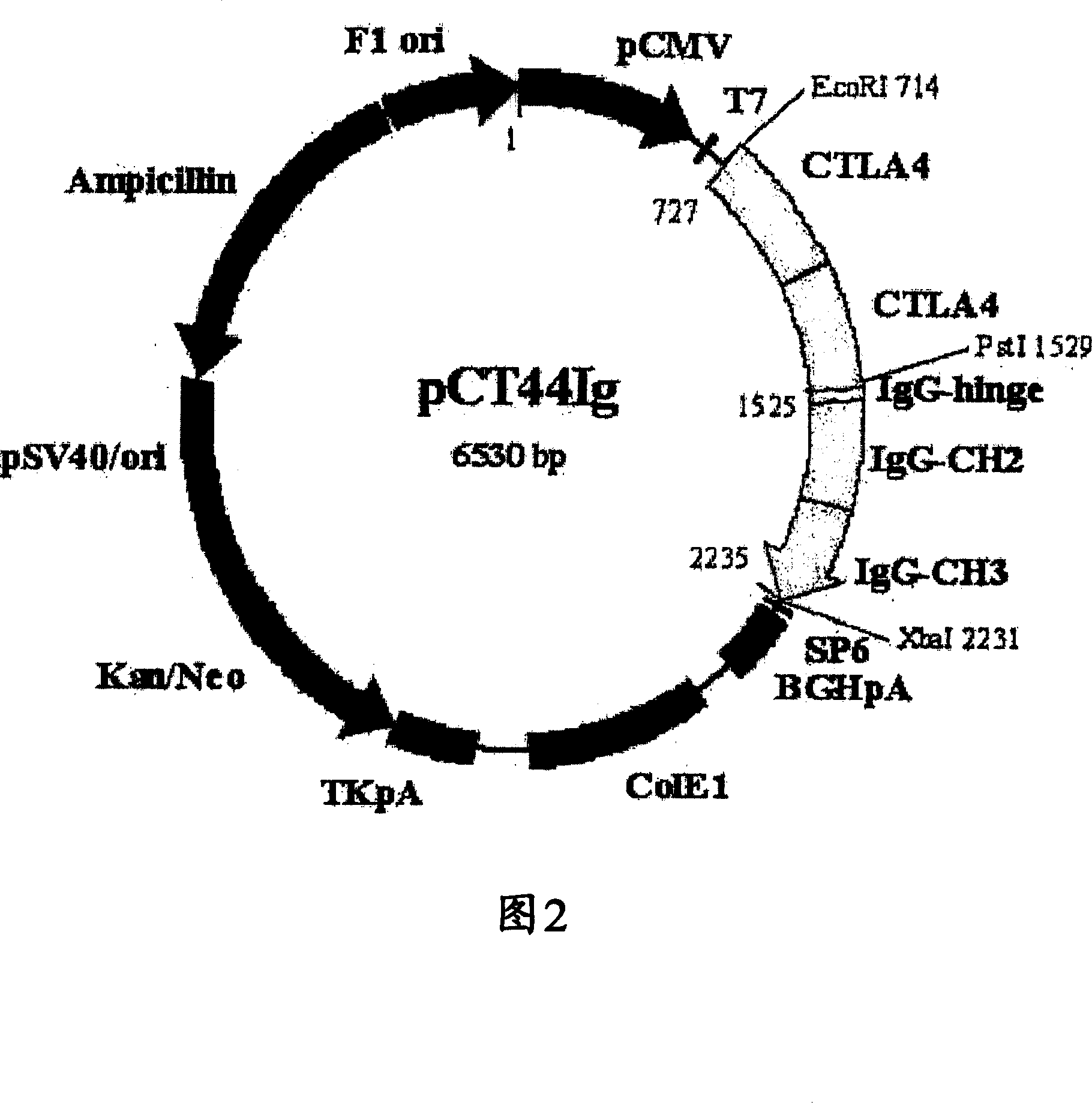
[0101] Simple fusion dimer proteins of other proteins TNFR1, TNFR2, CD2 and CTLA4 and linking fusion dimer proteins were prepared according to the same procedure as in Example 1. Detailed steps are described in PCT Publication No. WO2003 / 010202, which was filed by the present inventors. Information on the DNA and amino acid sequences of the Ig fusion proteins of TNFR1, TNFR2, CD2 and CTLA4 is summarized in Table 2 below.
[0102] Serial ID number
Embodiment 3
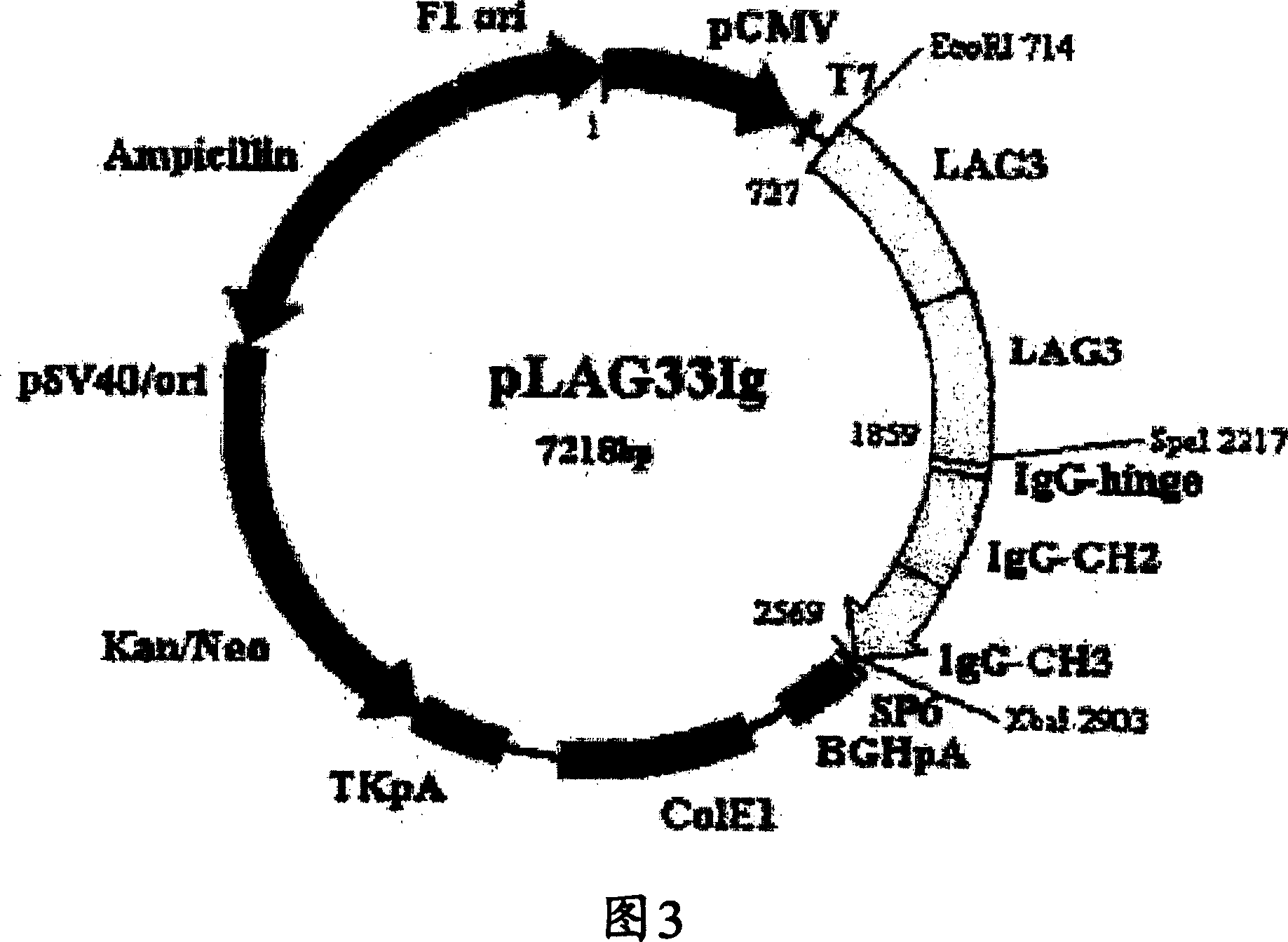
[0103] Example 3: Expression and Purification of Simple / Linked Fusion Dimeric Protein LAG3 / Fc
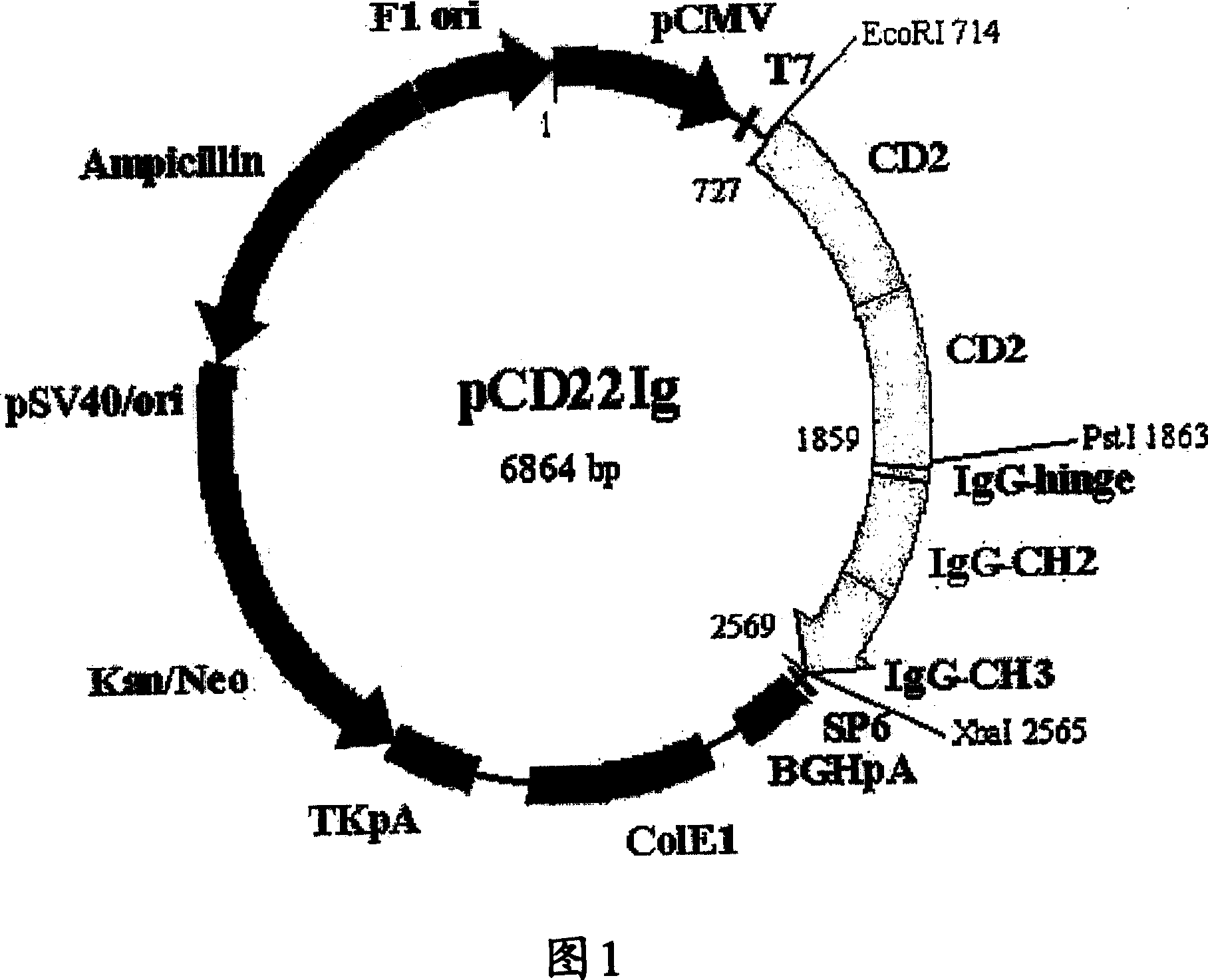
[0104] For expression of the fusion protein in CHO-K1 cells (ATCC CCL-61, ovary, Chinese hamster, Cricetulus griseus), pBluescript KS II(+) plasmid DNA containing the LAG3-LAG3 / Fc fusion gene was purified from transformed E. coli Afterwards, the LAG3-LAG3 / Fc fragment generated by restriction cutting with EcoRI and XbaI was expressed in the animal expression vector pCR TM 3 (Invitrogen, USA) was inserted into the EcoRI / XbaI site of the plasmid to construct an animal cell expression vector. These were designated as plasmid pLAG3-TOP10', and were deposited with the Korean Culture Center for Microorganisms (KCCM, 361-221, Yurim B / D, Hongje-1-dong, Seodaemun-gu, SEOUL 120-091, Korea).
[0105] The plasmid pLAG33IgDNA containing the LAG3-LAG3 / Fc fusion gene as described above was mixed with Lipofectamin TM (Gibco BRL, USA) reagents were mixed for transfection. CHO-K1 cells were treate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com