HLA-A2 restriction epi polypeptide originated from post selection cancer gene hRabj and its application
A technology of HLA-A2 and epitope polypeptides, which is applied to the HLA-A2 restricted epitope polypeptides derived from the new candidate oncogene hRabJ and its application field, which can solve the problems of lack of effective prevention and treatment of anti-tumor therapeutic vaccines and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
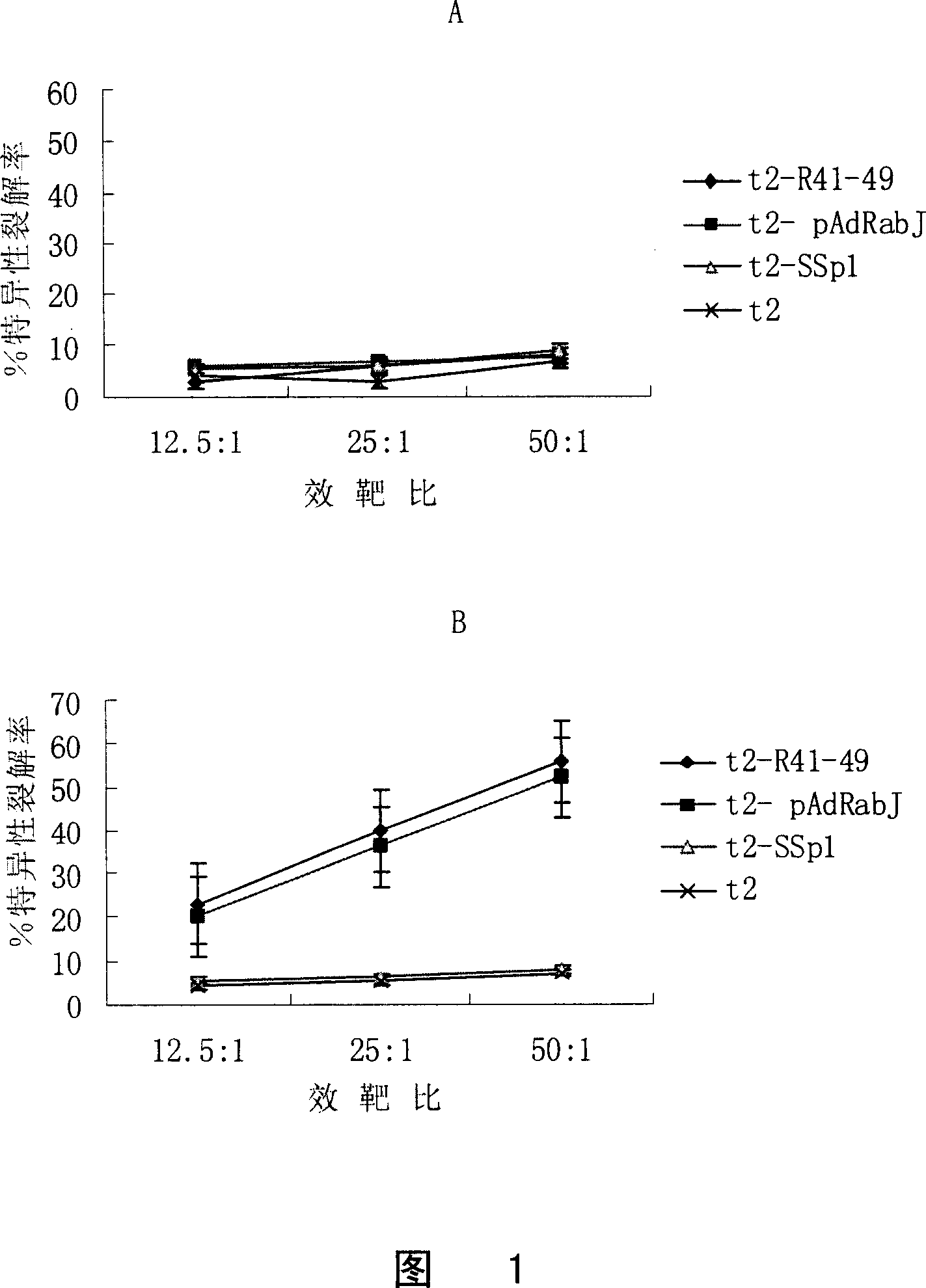
[0057] Example 1HLA-A * 0201 Screening of high affinity peptides
[0058] In this example, the peptides with HLA-A were screened out through peptide binding experiments.* 0201 high affinity epitope peptide.
[0059] experiment procedure
[0060] First collect T2 cells (a cell lacking antigen processing ability, purchased from ATCC: CRL-1991), wash three times with serum-free 1640, adjust the cell concentration to 2×10 5 cells / ml, spread in 24-well plate, 0.5ml / well. Then mix with 50μM candidate peptide, 2.5μg / ml β2 microglobulin at 37℃, 5% CO 2 A total of 18 hours of incubation in the incubator. The incubated cells were washed three times with ice-cold PBS, FITC-labeled HLA-A2-specific mAb BB7.2 (purchased from Sterotec Ltd, Oxford, UK) was added, ice-bathed for 45 minutes, washed with PBS and detected by flow cytometry mean fluorescence intensity. A positive known peptide CEA HLA-A2 restricted epitope polypeptide CAP-1 (SEQ ID NO: 10, sequence YLSGANLNL) was used as a p...
Embodiment 2
[0068] Embodiment 2 Preparation of adenovirus (pAdRabJ) expressing RabJ
[0069] Using the plasmid containing human RabJ cDNA obtained in Example 1 of Chinese patent 01126826.3 as a template, digested with SalI-XhoI, recombined with plasmid pShuttle-CMV (Stratagene Company) according to conventional methods, and transformed into competent Escherichia coli BL21, The positive clones were picked and identified, purified and sequenced (ABI company, BigDye Terminator kit). The human RabJ-pShuttle-CMV vector with the correct sequence was linearized with PmeI and co-transformed into Escherichia coli BJ5183 (Stratagene) with the pAdeasy adenovirus backbone plasmid (Stratagene). Positive clones were identified by PacI digestion, and the products were analyzed by 0.8% agarose gel electrophoresis. It was confirmed by sequencing that the adenoviral vector containing the coding sequence of human RabJ had been recombinantly produced.
[0070] After the adenoviral vector was linearized wit...
Embodiment 3
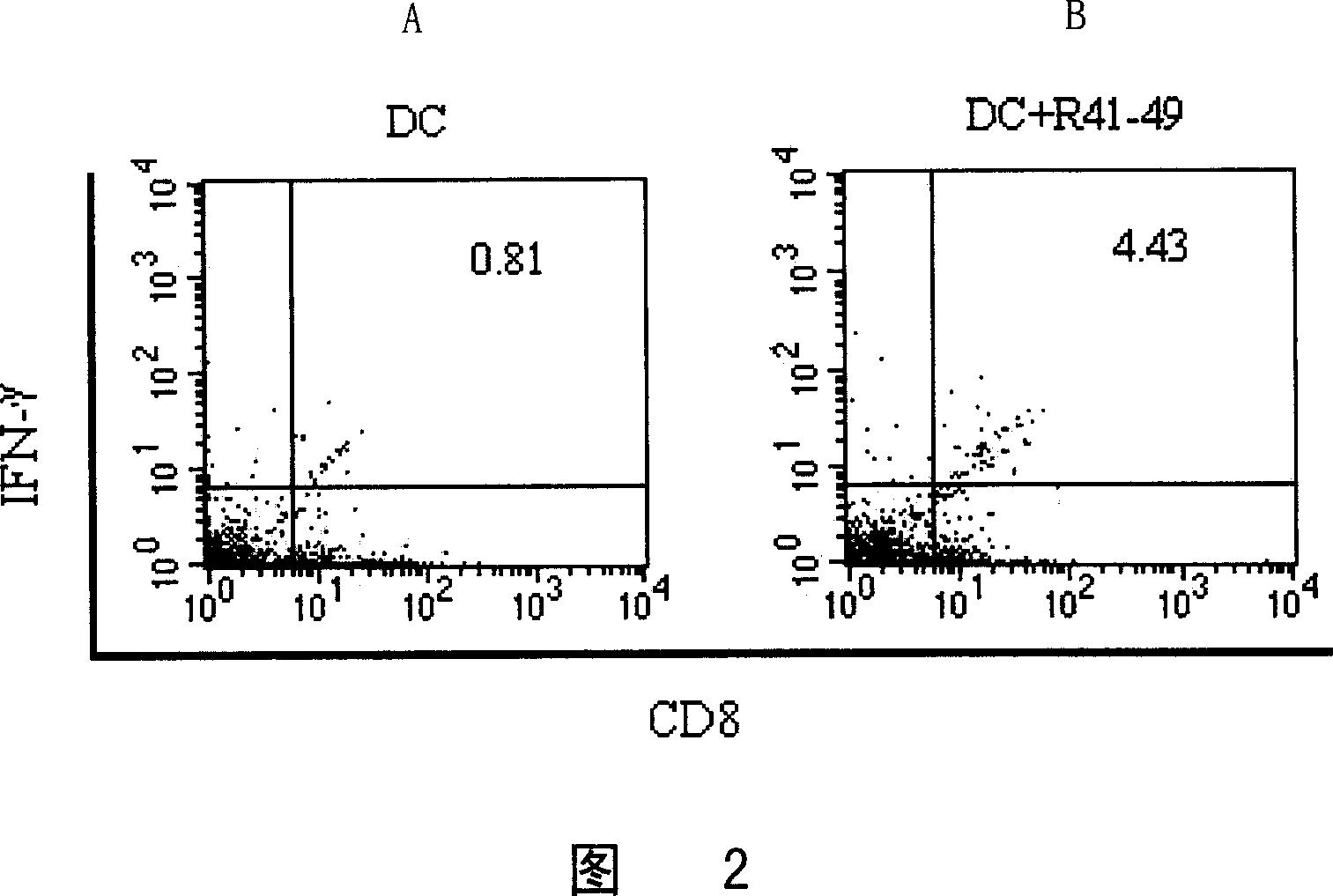
[0071] Example 3HLA-A2.1 / K b Induction of hRabJ-derived HLA-A2-restricted polypeptide-specific cytotoxic T lymphocytes in transgenic mice
[0072] Preparation of effector cells:
[0073] Prepare HLA-A2.1 / K by conventional method b Dendritic cells (DC) derived from bone marrow of transgenic mice. Collect the DC cultured to the 7th day, and adjust the cell concentration to 1×10 6 cells / ml, add R41-49 (final concentration 10 μM / ml) and β2 microglobulin (final concentration 3 μg / ml), 37°C, 5% CO 2 Incubate for 3 h in the incubator. Collect the peptide-sensitized DCs, wash them three times with PBS, and adjust the cell concentration to 1×10 6 cells / 0.2ml to immunize mice. Each mouse was injected intraperitoneally with 1×10 6 cells / 0.2ml, a total of three immunizations, with an interval of one week. Seven days after the last immunization, the mouse spleen was removed aseptically, and the red blood cells were dissolved to make a single cell suspension. Splenocyte suspension ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com