Preparation method of tissue engineering bone cartilage compound and its application
A tissue-engineered bone and composite technology, used in biochemical equipment and methods, bone implants, tissue cell/virus culture devices, etc., to achieve the effects of promoting early formation, delaying degeneration, and cartilage stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
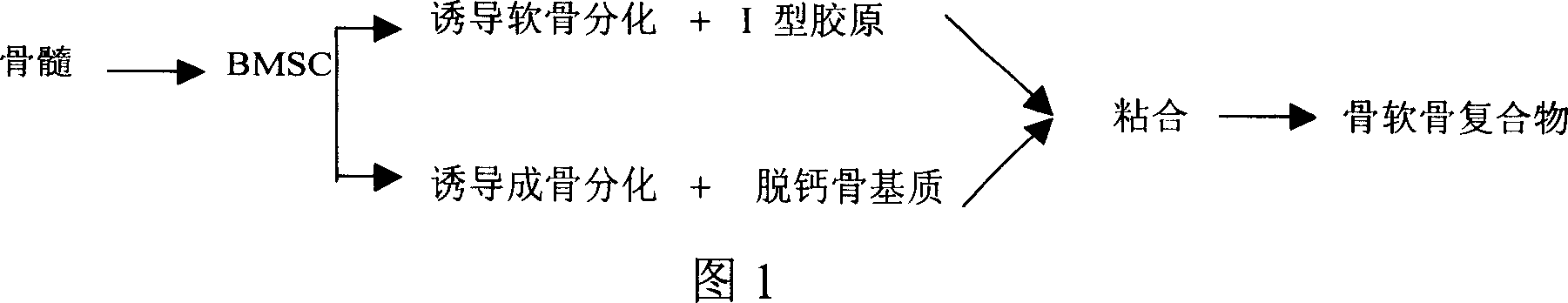
[0038] As shown in Figure 1, the tissue engineering osteochondral compound preparation method of the present invention comprises the following steps:
[0039]1) Use the method of density gradient centrifugation of lymphocyte separation medium to isolate and expand bone marrow pluripotent stem cells BMSCs in vitro. When BMSCs reach confluence at passage 2-3, digest and count according to 3×10 7 -6×10 7 / 100mm 2 Density inoculation, using different cartilage and osteogenesis induction systems for initial induction of cartilage and bone origin;
[0040] 2) taking fresh ilium and knee joints of New Zealand rabbits, and performing decalcification treatment to prepare decalcified bone matrix;
[0041] 3) Then the demineralized bone matrix and type I collagen sponge were cut into cubes of required size, pre-coated with human fibronectin and dried overnight;
[0042] 4) BMSCs cultured in different induction systems in step 1 were respectively inoculated into the decalcified bone ma...
Embodiment 1
[0046] Example 1 Using mesenchymal stem cells to prepare tissue-engineered cartilage to repair articular cartilage defects
[0047] 1) In vitro isolation and expansion of bone marrow pluripotent stem cells: extract rabbit bone marrow through bone marrow puncture, and use density gradient centrifugation to separate mononuclear cells with lymphocyte separation medium (specific gravity: 1.077) 7 / 100mm 2 The density was inoculated in culture flasks, and the culture medium contained 60% DMEM-LG / F / 12, 40% MCDB-201 (Gibco), 2% FBS, 10ng / ml EGF, 10ng / ml PDGF-bb (Peprotech) components . Place at 37°C, 5% CO 2 cultured in an incubator. After 24 to 72 hours, discard the suspended cells, add fresh medium to continue culturing, and change the medium every 3 days until the cells grow to 80% confluence, digest the cells with 0.25% trypsin-0.1% EDTA at 37°C, Count to 3 x 10 4 -5×10 4 / cm 2 Cells were inoculated at a certain density, and cultured and subcultured according to the above ...
Embodiment 2
[0052] Example 2 Using Bone Marrow Mesenchymal Stem Cells to Prepare Tissue-Engineered Osteochondral Compounds to Repair Articular Cartilage Defects
[0053] 1) In vitro isolation and expansion of bone marrow pluripotent stem cells: the rabbit bone marrow was extracted by bone marrow puncture, and the bone marrow mononuclear cells were separated by lymphocyte separation medium. 7 / 100mm 2 The density was inoculated in culture flasks, and the culture medium contained 60% DMEM-LG / F / 12, 40% MCDB-201 (Gibco), 2% FBS, 10ng / ml EGF, 10ng / ml PDGF-bb (Peprotech) components . Place at 37°C, 5% CO 2 cultured in an incubator. After 2-3 days, discard the suspended cells, add fresh medium to continue culturing, and change the medium every 3 days until the cells grow to 80% confluence, digest the cells with 0.25% trypsin-0.1% EDTA at 37°C, and count , to 3 x 10 4 -5×10 4 / cm 2 Cells were inoculated at a certain density, and cultured and subcultured according to the above conditions. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com