Method for preparing potassium ferrate by using waste liquid from acid washing steel
A technology for iron and steel pickling waste liquid, potassium ferrate, applied in chemical instruments and methods, iron compounds, sodium/potassium compounds, etc., can solve problems such as environmental pollution and resource waste, achieve a wide range of use, eliminate pollution, and The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
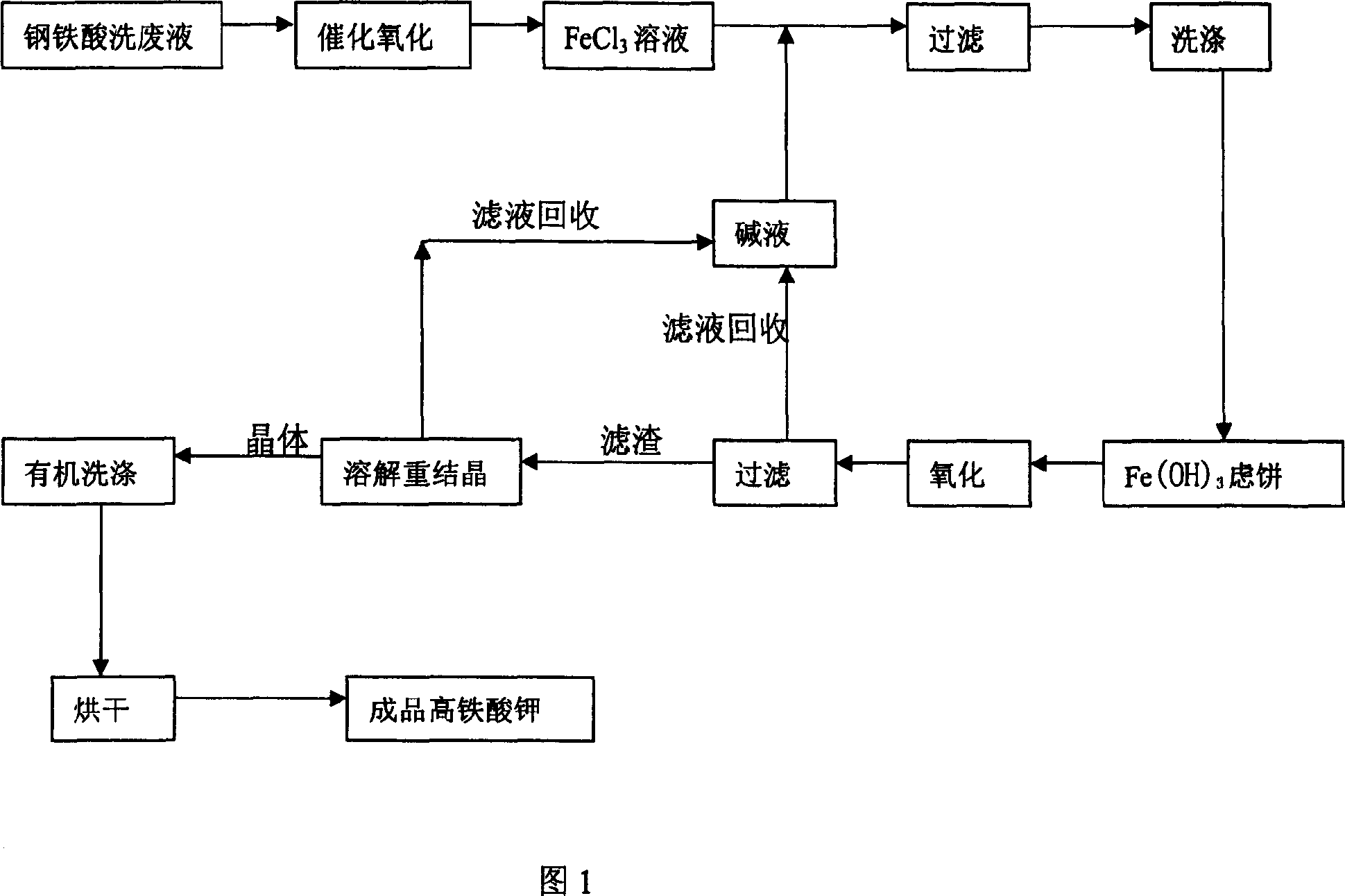
Image
Examples
Embodiment 1
[0022] A method for preparing potassium ferrate by utilizing iron and steel pickling waste liquor, it comprises the steps:
[0023] 1) Iron and steel pickling waste liquid (containing Fe 2+ The mass percentage is 0.1%, Fe 3+ The mass percentage is 0.1%, Cl - The mass percentage is 0.1%, and the acidity is 0mol / l) and the hydrochloric acid that the mass fraction is 21% is mixed with the volume ratio of 1000:6, and the mixed solution is aerated with an aerator, and heated to 40°C; when the temperature reaches 40°C , add NaNO with a weight concentration of 1‰ 2 , NaNO with a weight concentration of 1‰ 2 The dosage is 0.1% of the mass of the mixed liquid, continue to aerate, and maintain the system at 40°C for 1 hour; the reaction equation involved:
[0024] ,
[0025] ,
[0026] After the reaction was completed, the reaction solution was added to the alkali solution, and the Fe 3+ with Fe(OH) 3 Precipitated in the form of, among them, OH - The total amount of...
Embodiment 2
[0033] A method for preparing potassium ferrate by utilizing iron and steel pickling waste liquor, it comprises the steps:
[0034] 1) Iron and steel pickling waste liquid (containing Fe 2+ The mass percentage is 10%, Fe 3+ The mass percentage is 10%, Cl - The mass percentage is 20%, and the acidity is 3mol / l) and the hydrochloric acid that the mass fraction is 10% is mixed with the volume ratio of 1000:30, and the mixed solution is aerated with an aerator, and heated to 70°C; when the temperature reaches 70°C , add NaNO with a weight concentration of 30‰ 2 , NaNO with a weight concentration of 30‰ 2 The dosage is 2% of the mass of the mixture, continue to aerate, and maintain the system at 70°C for 4 hours; the reaction equation involved:
[0035] ,
[0036] ,
[0037] After the reaction was completed, the reaction solution was added to the alkali solution, and the Fe 3+ with Fe(OH) 3 Precipitated in the form of, among them, OH - The total amount of matte...
Embodiment 3
[0044] A method for preparing potassium ferrate by utilizing iron and steel pickling waste liquor, it comprises the steps:
[0045] 1) Iron and steel pickling waste liquid (containing Fe 2+ The mass percentage is 20%, Fe 3+ The mass percentage is 20%, Cl - The mass percentage is 35%, acidity 6mol / l) is mixed with the hydrochloric acid that mass fraction is 21% with the volume ratio of 1000:40, the mixed solution is aerated with an aerator, and heated to 90 ℃; when the temperature reaches 90 ℃ , add NaNO with a weight concentration of 50‰ 2 , NaNO with a weight concentration of 50‰ 2 The dosage is 5% of the mass of the mixed liquid, continue to aerate, and maintain the system at 90°C for 6 hours; the reaction equation involved:
[0046] ,
[0047] ,
[0048] After the reaction was completed, the reaction solution was added to the alkali solution, and the Fe 3+ with Fe(OH) 3 Precipitated in the form of, among them, OH - The total amount of matter is Fe 3+ 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com