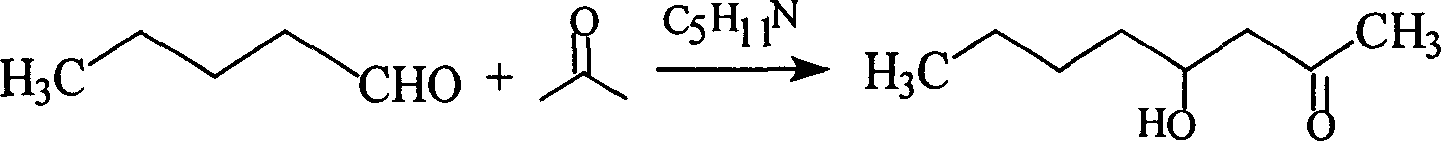Method for synthesis of 3-octene-2-ketone
A synthesis method and technology of octene, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of complex process and low product yield, and achieve the effect of high yield, less by-products and good dehydration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0008] The implementation of the present invention will be further described below.
[0009] The first step: the preparation of 4-hydroxy-2-octanone.
[0010] Add 135g (2.33mol) of acetone, 6g (0.10mol) of acetic acid, and 2g (0.024mol) of piperidine into a 500ml round-bottomed three-necked flask equipped with a stirrer, dropping funnel, and internal thermometer. Stirring was started, and 50 g (0.58 mol) of valeraldehyde was added dropwise at about 15°C, and the addition was completed in about 1 hour, and the reaction was continued at room temperature for 3-5 hours. Stirring was stopped, and acetone was recovered under normal pressure. Distilled under reduced pressure to obtain 71 g of the product 4-hydroxy-2-octanone with a content of 95% and a yield of 85%.
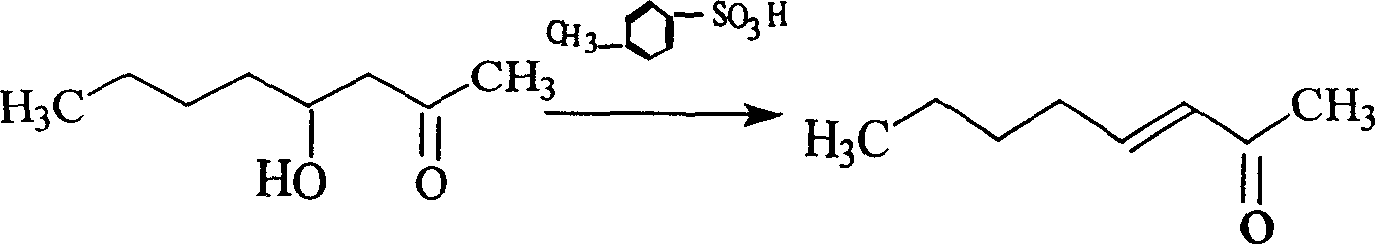
[0011] The second step: the preparation of 3-octen-2-one.
[0012] Add 72g (0.5mol) of 4-hydroxyl-2-octanone, 200ml of cyclohexane, and 1g of p-toluenesulfonic acid in a 500ml round-bottomed three-necked flask equipp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com