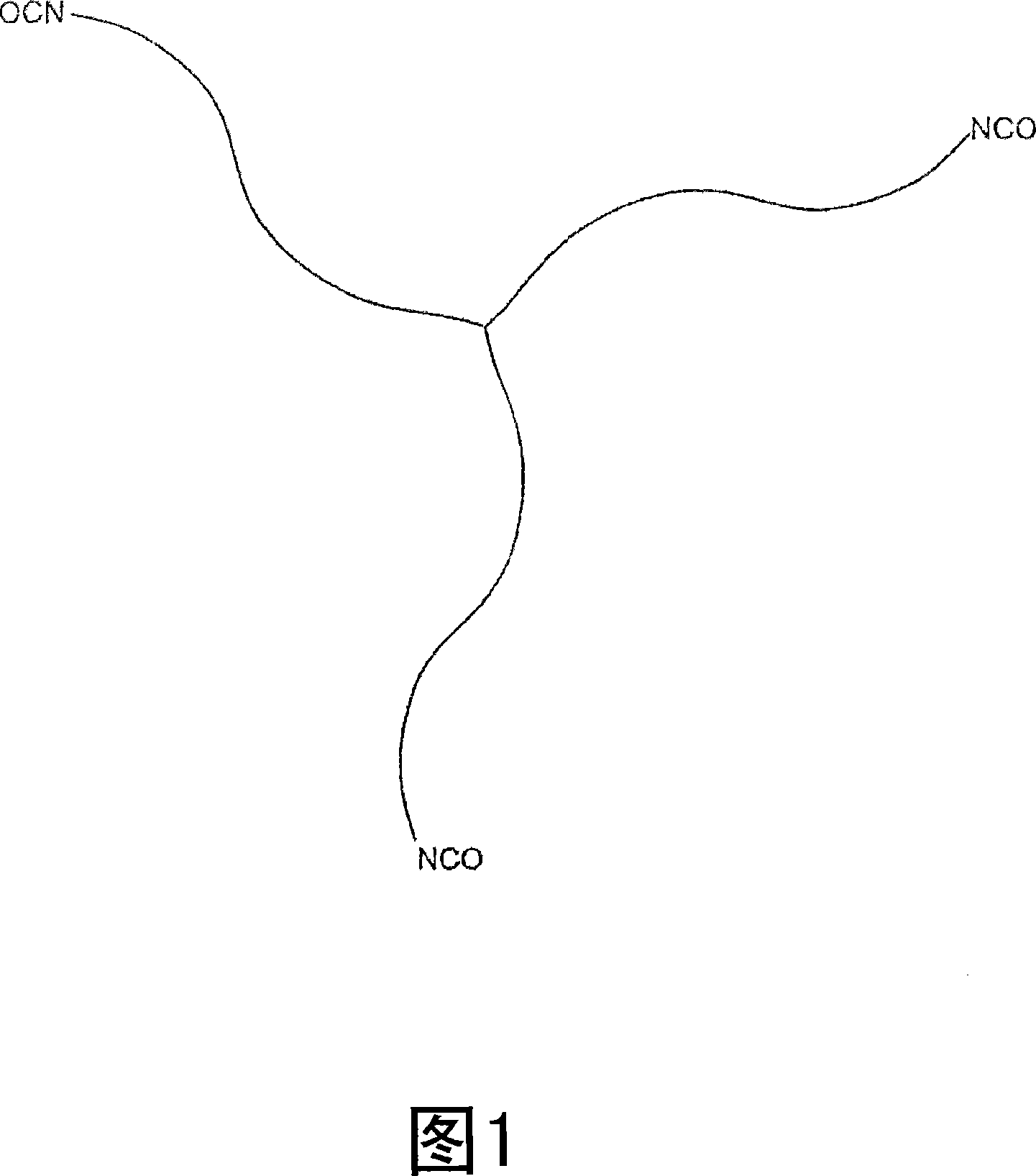
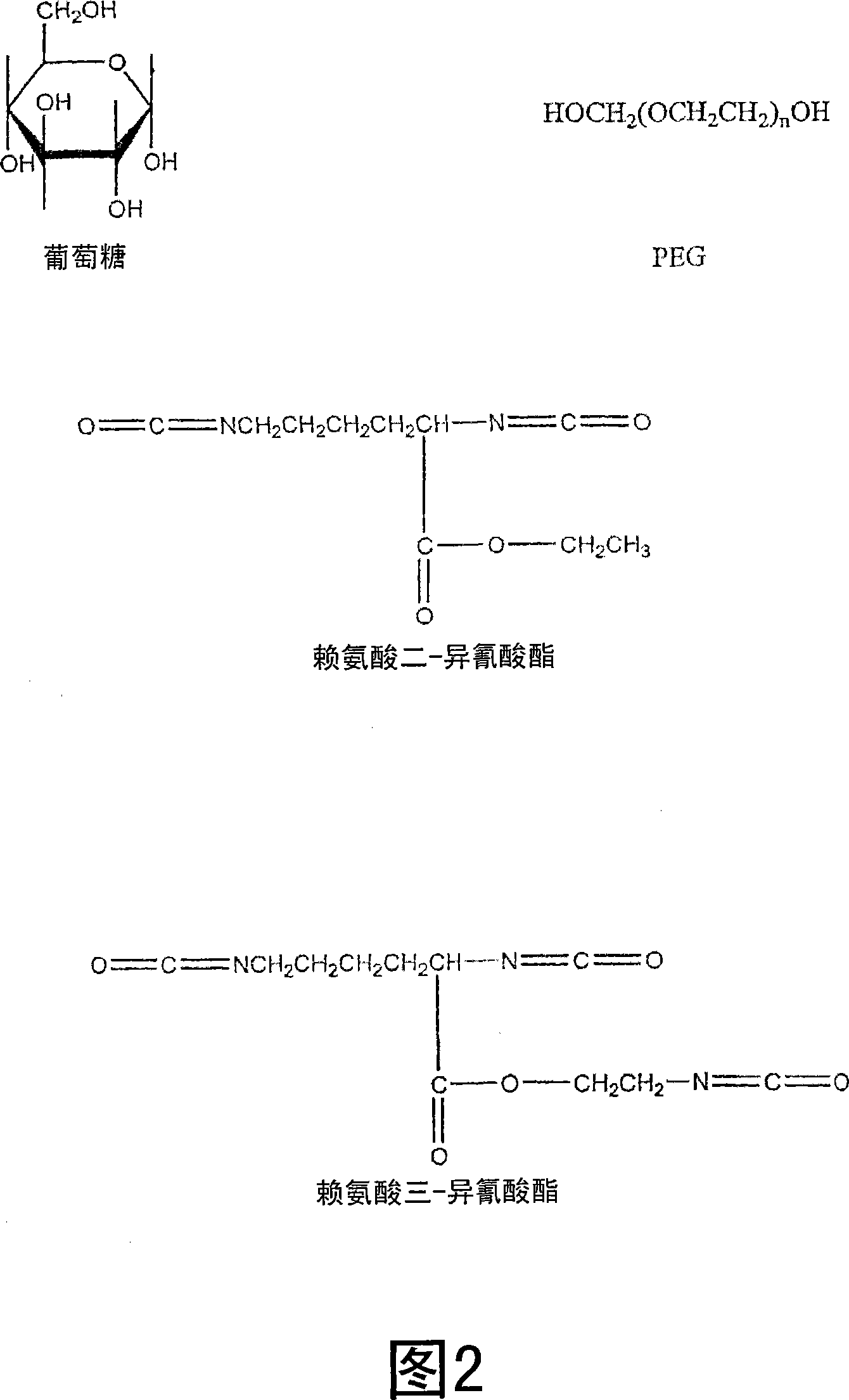
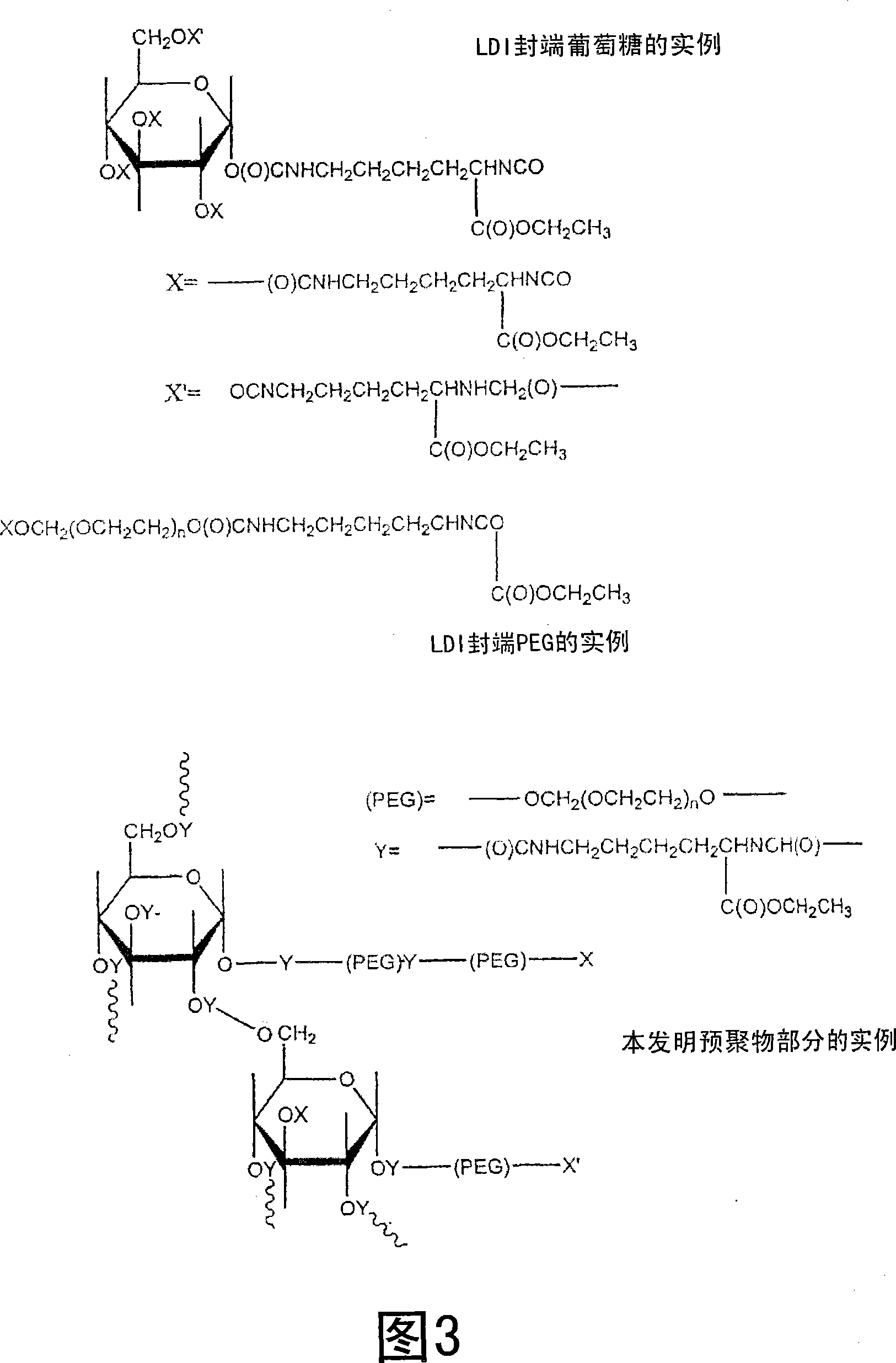
Medical adhesive and methods of tissue adhesion
A technology of adhesives and organic tissues, applied in the direction of surgical adhesives, applications, medical science, etc., to achieve the effects of easy synthesis, reduced possibility, and strong tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Representative LDI-based polyurethane tissue adhesives or stickies were synthesized using the procedure described below. To prepare the adhesive, 0.5889 g of glucose (3.27 mmol, -OH 16.36 mmol) was added to 5 ml of PEG 400 (14.09 mmol, -OH 28.18 mmol) in a dry round bottom flask, purged with nitrogen and heated at 50 Heated at ℃ to obtain a transparent solution. PEG is liquid at room temperature and dissolves glucose without the need for additional solvents. Then, 4.6 ml of lysine di-isocyanate (LDI, d 1.157, FW 226, 23.55 mmol, -NCO 47.10 mmol) were added, and the flask was fitted with a rubber septum and sealed. The reaction mixture was stirred at 50 °C for 48 hrs, resulting in a viscous solution. Store the viscous solution under nitrogen at room temperature until use. The viscous liquid is spread on each of the two wet tissues, and when the two tissues are pressed together, they adhere firmly to each other after about 1-2 minutes.
Embodiment 2
[0047] Using PEG200 instead of PEG400, another LDI-based polyurethane tissue adhesive was synthesized using the procedure described below, which ultimately resulted in a harder, stronger seal than the adhesive of Example 1. During this process, 0.6 g of glucose (3 mmol, -OH 15 mmol) was added to 5 ml of PEG 200 (28.18 mmol, -OH 56.35 mmol) in a dry round bottom flask, purged with nitrogen and heated at 50 °C, A clear solution was obtained. Then, 7ml of LDI (d 1.157, FW 226, 35.83mmol, -NCO 71.67mmol) was added, and the flask was fitted with a rubber septum and sealed. The reaction mixture was stirred at 50 °C for 48 hrs, resulting in a viscous solution. Store stickies under nitrogen at room temperature until use. Spread the viscous liquid over each of the two wet tissues, and when the two tissues are pressed together, they adhere firmly to each other after 1-2 minutes.
Embodiment 3
[0049] Example 3 shows that when the glucose fraction in the reaction mixture is increased, the time required to close the wound is shorter, the bond strength is increased and the final material is stiffer. In this study, 1.8 g of glucose (10 mmol, -OH 50 mmol) was added to 5 ml of PEG 200 (28.18 mmol, -OH 56.35 mmol) in a dry round bottom flask, purged with nitrogen and heated at 50 °C, A clear solution was obtained. Then, 10 ml of LDI (d 1.157, FW 226, 51.19 mmol, -NCO 102.02 mmol) were added. The flask was fitted with a rubber septum and sealed. The reaction mixture was stirred at 50 °C for 48 hrs, resulting in a viscous solution. The stickies were stored under nitrogen at room temperature until use. The viscous liquid was allowed to spread over each of the two wet tissues, and when the two tissues were pressed together, they adhered firmly to each other after about 1 minute.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Functional group degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com