Filter and cyanine compound
A filter and compound technology, applied in the fields of filter, optics, organic chemistry, etc., to achieve the effects of excellent light resistance and solubility, high molar absorption coefficient, and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
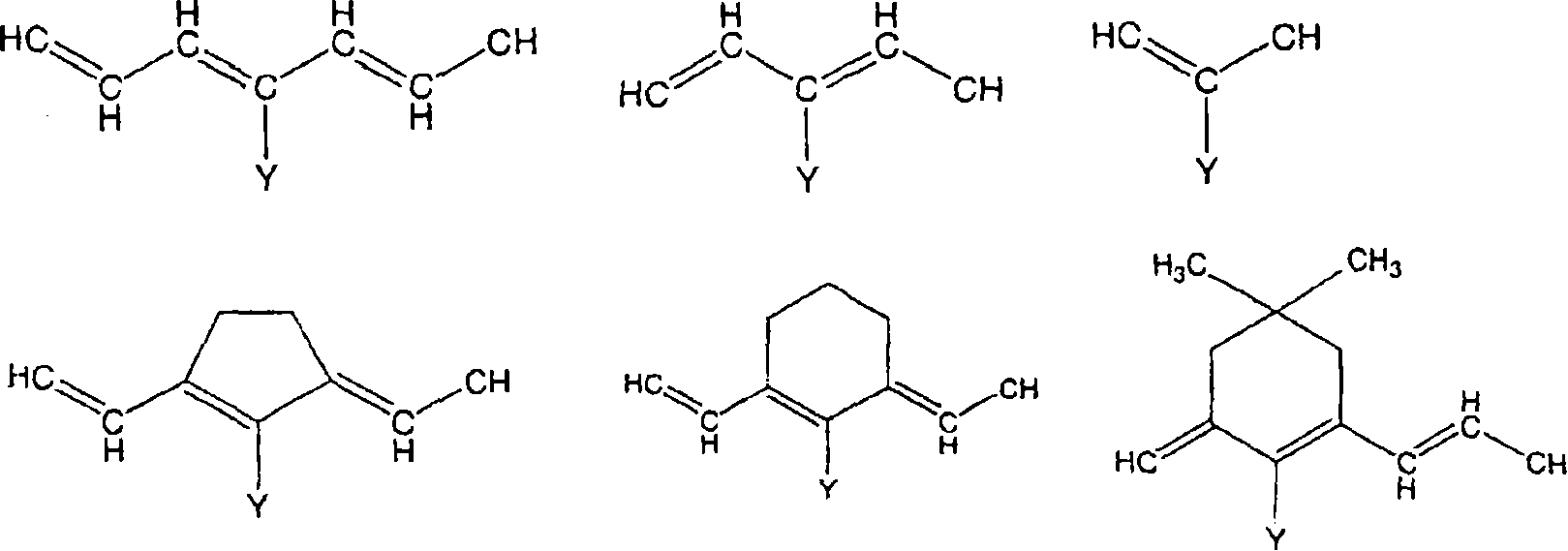
Method used
Image
Examples
Embodiment 1
[0092] Synthesis of compound 1
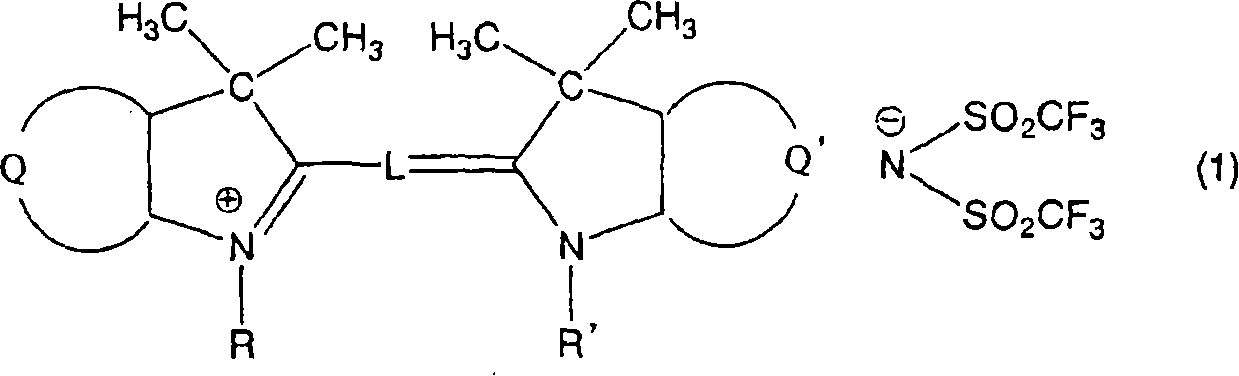
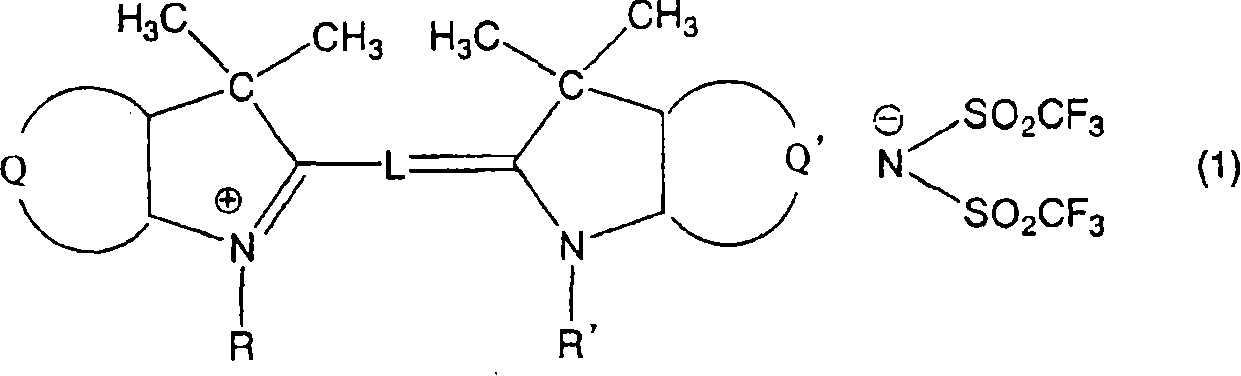
[0093] Mix 9.6 parts of 1,3,3-trimethyl-2-methyleneindoline with 10.6 parts of 1,3,3-trimethyl-2-formylmethyleneindoline, 17.6 parts of (bistrifluoromethanesulfonyl) shown in the formula
[0094]
[0095] Potassium imide was placed in 75 parts of acetic anhydride, boiled for 1 hour under reflux condensation, then cooled to room temperature, and the reaction solution was suction filtered to remove insoluble impurities. Then, 100 parts of water was dropped into the reaction solution, the precipitated crystals were suction filtered, 40 parts of methanol were added for recrystallization, washed with 5 parts of methanol, washed with water and dried to obtain 19.4 parts of the aforementioned compound 1. The spectral characteristics of the obtained compound 1 are as follows.
[0096] The maximum absorption wavelength is 544nm (in methanol)
[0097] Molar absorptivity 133000 (in methanol)
Embodiment 2
[0099] Synthesis of compound 3
[0100] 14.9 parts of 4,5-benzo-1-(2-methoxyethyl)-3,3-dimethyl-2-methylene indoline, 8.9 parts of triethyl orthoformate, 8.8 Parts of (bistrifluoromethanesulfonyl) potassium imide, 2.5 parts of concentrated hydrochloric acid were placed in a mixed solvent of 50 parts of acetic anhydride and 25 parts of acetic acid, boiled for 2 hours under reflux condensation, then cooled to room temperature, added 25 parts of water, suction-filter the precipitated crystals, boil in 15 parts of isopropanol under reflux condensation for 1 hour, then cool to room temperature, suction-filter the crystals, wash with 5 parts of isopropanol, then wash with water, After drying, 13.4 parts of the aforementioned compound 3 were obtained. The spectral characteristics of the obtained compound 3 are as follows.
[0101] Maximum absorption wavelength 588nm (in methanol)
[0102] Molar absorptivity 119000 (in methanol)
Embodiment 3
[0104] Synthesis of Compound 12
[0105] Divided by 14.9 parts of 4,5-benzo-1-(2-methoxyethyl)-3,3-dimethyl-2-methyleneindoline to replace 9.6 parts of 1,3,3- Except for trimethyl-2-methylene indoline, 18.3 parts of the aforementioned compound 12 were obtained in the same manner as in Example 1. The spectral characteristics of the obtained compound 12 are as follows.
[0106] Maximum absorption wavelength 555nm (in methanol)
[0107] Molar absorptivity 119000 (in methanol)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com