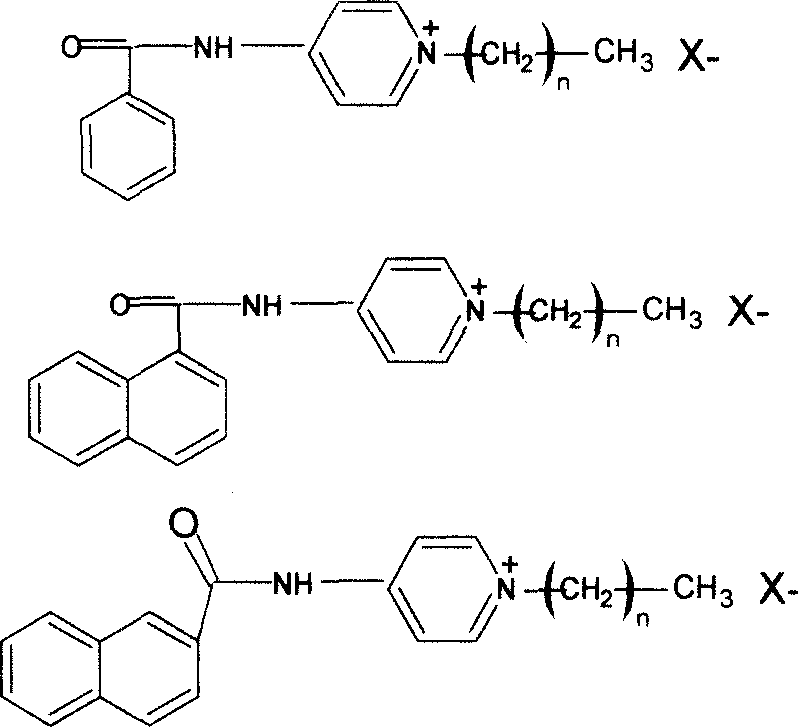
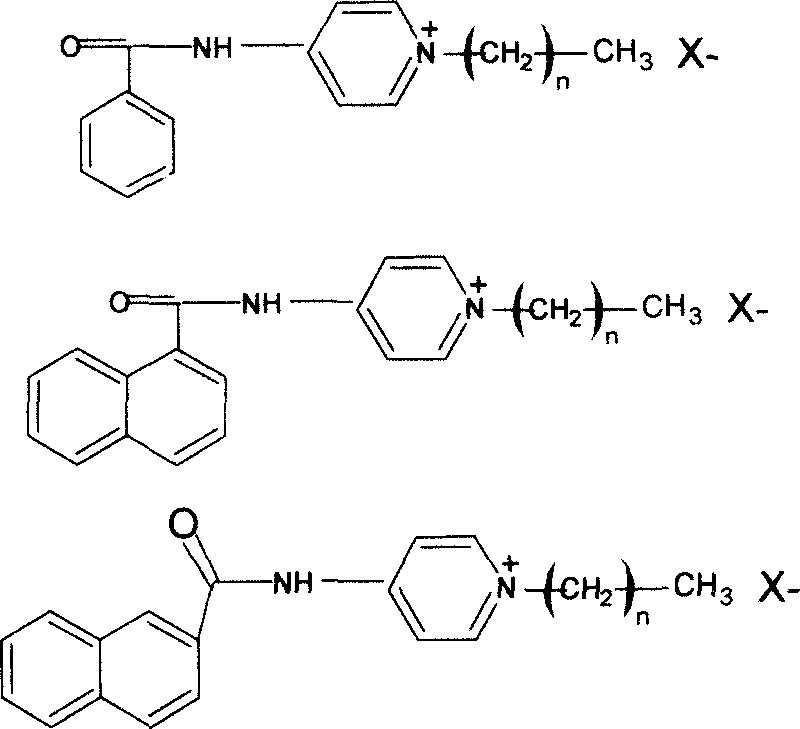
Pyridyl quaternary ammonium salt antiseptic and its prepn process
A technology of pyridinium quaternary ammonium salt and antibacterial agent, which is applied in the field of pyridinium quaternary ammonium salt antibacterial agent and its preparation, can solve the problems that the structure and antibacterial property of quaternary ammonium salt have not been studied in detail, achieve good bactericidal effect, simple preparation method, The effect of high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put a mixture of 10g of benzoyl chloride and 50ml of acetone into a 250ml three-neck flask, cool it in ice water to 0-5°C, dissolve 5g of 4-aminopyridine in an appropriate amount of acetone, and stir it within 30min Continuously add to the benzoyl chloride solution, keep the temperature and continue the reaction for 1 hour, after completion, remove the acetone solution to obtain white crystals of benzamidopyridine, which are recrystallized in water. Dry in a vacuum oven. The yield is about 93%.
[0020] Dissolve 7.8g of benzamidopyridine and 3.8g of dodecyl bromide in 100ml of DMSO, put them in a 250ml flask, heat the mixture in an oil bath at 100°C for about 4 hours, remove DMSO in a vacuum evaporator, The reaction product was washed with ether, and recrystallized in an ether-ethanol mixed solvent to obtain light yellow solid particles, which were then vacuum-dried in a vacuum dryer for more than 24 hours.
[0021] Gained pyridine quaternary ammonium antibacterial ag...
Embodiment 2
[0024] Put a mixture of 7.6g of 1-naphthoyl chloride and 80ml of acetone into a 250ml three-neck flask, cool it in ice water to 0-5°C, dissolve 3.8g of 4-aminopyridine in an appropriate amount of acetone, and stir It was continuously added to the 1-naphthoyl chloride solution within 30 minutes, and the temperature was maintained to continue the reaction for 1 hour. After completion, the acetone solution was removed to obtain white crystals of 1-naphthoylaminopyridine. Dry in a vacuum oven. The yield is about 92%.
[0025] Dissolve 6.4g of 1-naphthoylaminopyridine and 7.0g of dodecyl bromide in 100ml of DMSO, put them in a 250ml flask, heat the mixture in an oil bath at 100°C for about 4 hours, and remove it in a vacuum evaporator Wash the reaction product with DMSO and diethyl ether, and recrystallize in a diethyl ether-ethanol mixed solvent to obtain a light yellow solid particle, which is then vacuum-dried in a vacuum dryer for more than 24 hours.
[0026] Gained pyridine ...
Embodiment 3
[0029] Put a mixture of 7.6g of 2-naphthoyl chloride and 80ml of acetone into a 250ml three-neck flask, cool it in ice water to 0-5°C, dissolve 3.8g of 4-aminopyridine in an appropriate amount of acetone, and stir It was continuously added to the 1-naphthoyl chloride solution within 30 minutes, and the temperature was maintained to continue the reaction for 1 hour. After completion, the acetone solution was removed to obtain 1-naphthoylaminopyridine as white crystals. Dry in a vacuum oven. The yield is about 94%.
[0030] Dissolve 6.4g of 1-naphthoylaminopyridine and 7.0g of dodecyl bromide in 100ml of DMSO, put them in a 250ml flask, heat the mixture in an oil bath at 100°C for about 4 hours, and remove it in a vacuum evaporator The reaction product was washed with DMSO and diethyl ether, and recrystallized in a diethyl ether-ethanol mixed solvent to obtain light yellow solid particles, which were then vacuum-dried in a vacuum dryer for more than 24 hours.
[0031] Gained p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com