Fast releasing solid omeprazole prepn and its prepn process
A solid preparation, omeprazole technology, applied in the field of pharmaceutical preparations, can solve problems such as gastrointestinal damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
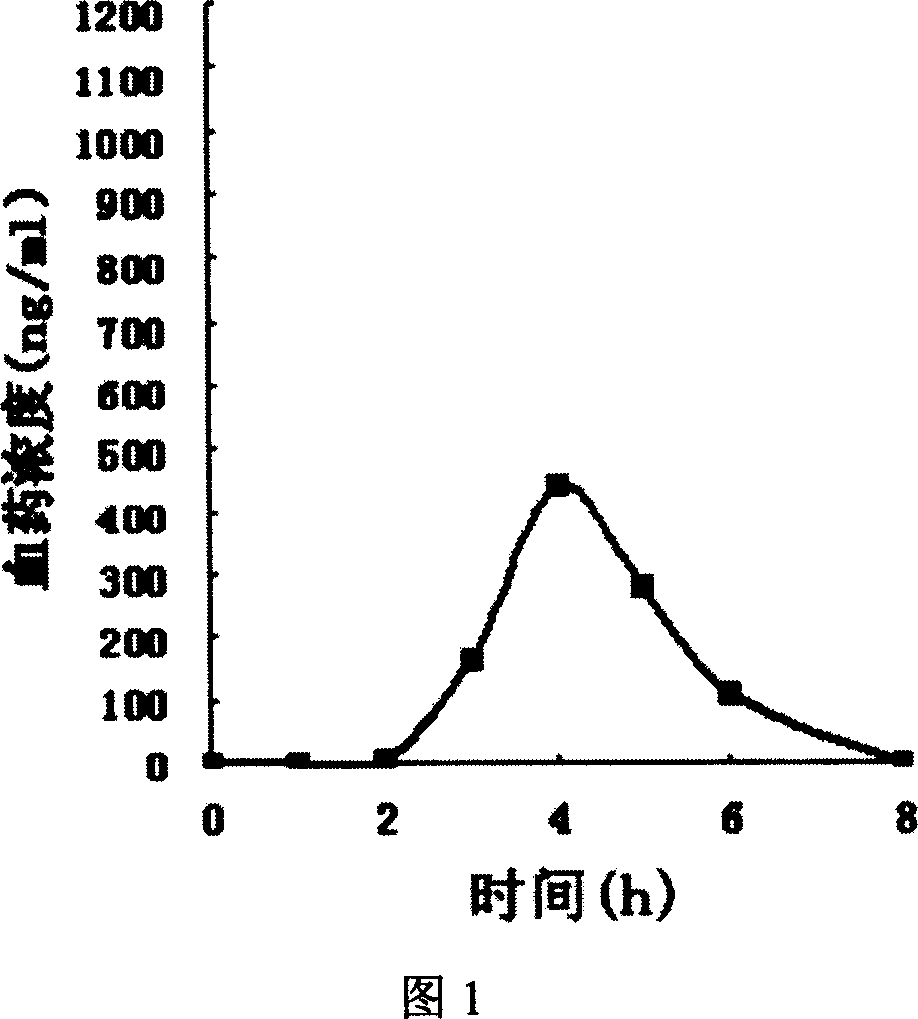
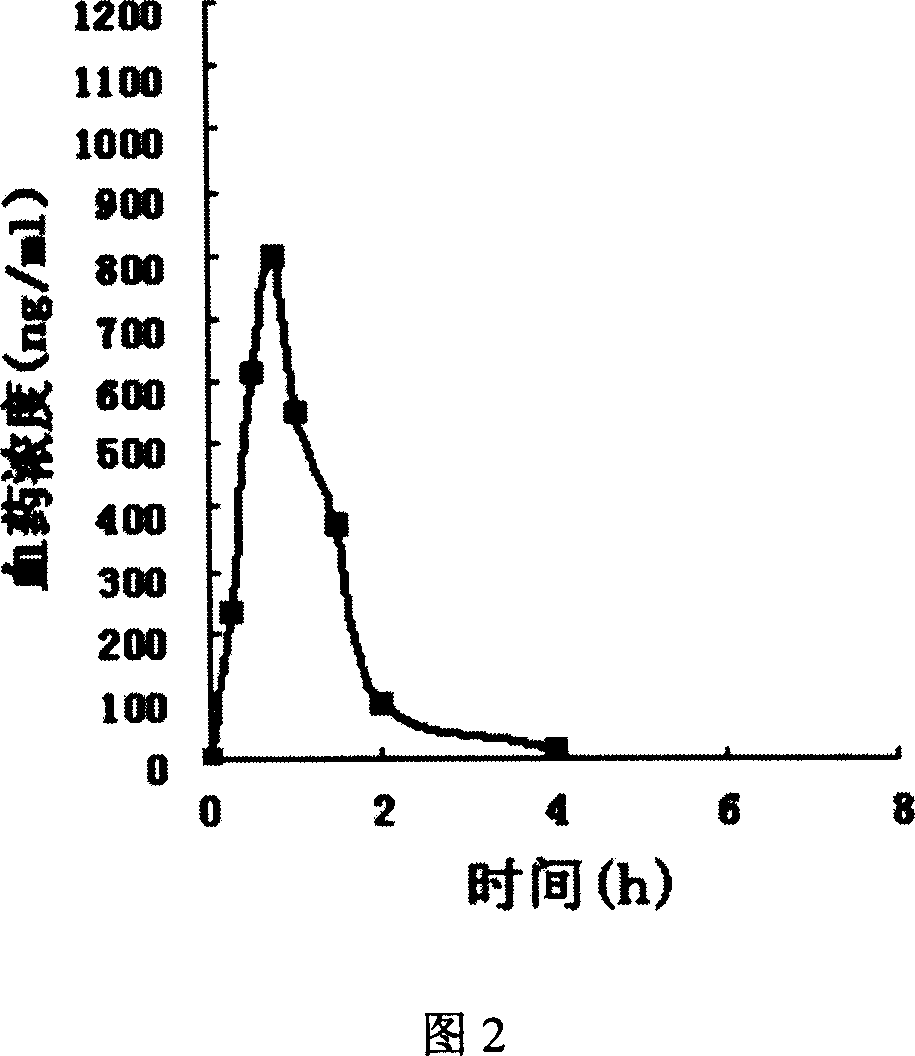
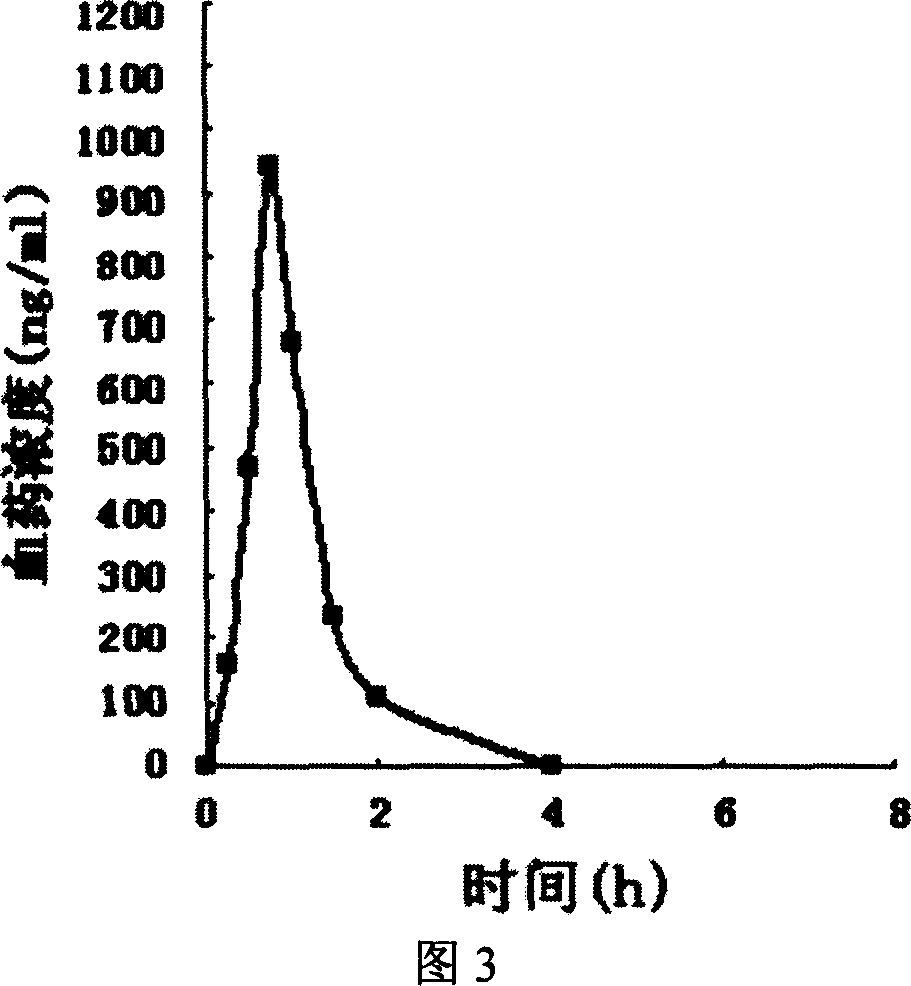
Image
Examples
Embodiment 1
[0062] Common tablets each containing omeprazole 10 mg and sodium hydroxide 350 mg were prepared.
[0063] The prescription is:
[0064] Omeprazole 1g
[0065] Sodium hydroxide 35g
[0066] Aluminum magnesium carbonate 100g
[0067] Total weight 136g
[0068] The preparation process is: (Operation in dark place, humidity control and temperature control, the same below)
[0069] Raw and auxiliary materials are pulverized through 80 mesh sieves, and the omeprazole, sodium hydroxide and aluminum magnesium carbonate of prescription quantity are taken and mixed evenly, and directly compressed into tablets, and 100 fast-disintegrating tablets are made altogether, each containing omeprazole 10mg, Sodium hydroxide 350mg, tablet diameter 1.6cm, hardness 4-5kg, store in a dry and low temperature place.
Embodiment 2
[0071] Prepare fast-disintegrating tablets each containing 10 mg of omeprazole and 400 mg of sodium hydroxide.
[0072] The prescription is:
[0073] Omegra 1g
[0074] Sodium hydroxide 40g
[0075] Calcium hydrogen phosphate 54g
[0076] Croscarmellose Sodium 15g
[0077] 2% Povidone Alcohol Solution QS
[0078] Total weight 110g
[0079] The preparation process is: (Operation in dark place, humidity control and temperature control, the same below)
[0080] Raw and auxiliary materials are crushed through an 80-mesh sieve, weighed omeprazole, sodium hydroxide, calcium hydrogen phosphate and 50% of the prescribed amount of croscarmellose sodium and mixed evenly, add 2% povidone alcohol solution Appropriate amount, granulate with a 24-mesh sieve, dry at 40°C for 5 hours, granulate with a 30-mesh sieve, add 50% of the prescription amount of croscarmellose sodium, mix evenly, and press into tablets to make a total of 100 rapidly disintegrating tablets , each tablet contains...
Embodiment 3
[0083] Each chip containing 20 mg of omeprazole and 600 mg of sodium hydroxide was prepared.
[0084] The prescription is:
[0085] Omeprazole 2g
[0086] Sodium hydroxide 60g
[0087] Cyclodextrin 30g
[0088] Cross-linked polyvinylpyrrolidone 40g
[0089] 2% Povidone Alcohol Solution QS
[0090] Total weight 100g
[0091] The preparation process is: (operation in dark place, the same below)
[0092] Raw and auxiliary materials are pulverized through 80 mesh sieves, and the omeprazole of prescription quantity, the sodium hydroxide of 50% prescription quantity and the cross-linked polyvinylpyrrolidone of 50% prescription quantity are taken by weighing, and the medicine-containing pill core is prepared, and the hydrogen of 50% prescription quantity is added. The sodium oxide, the cyclodextrin of the prescribed amount and the cross-linked polyvinylpyrrolidone of 50% of the prescribed amount are mixed uniformly, and directly compressed into tablets. Pack 100 chips, each co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gross weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com