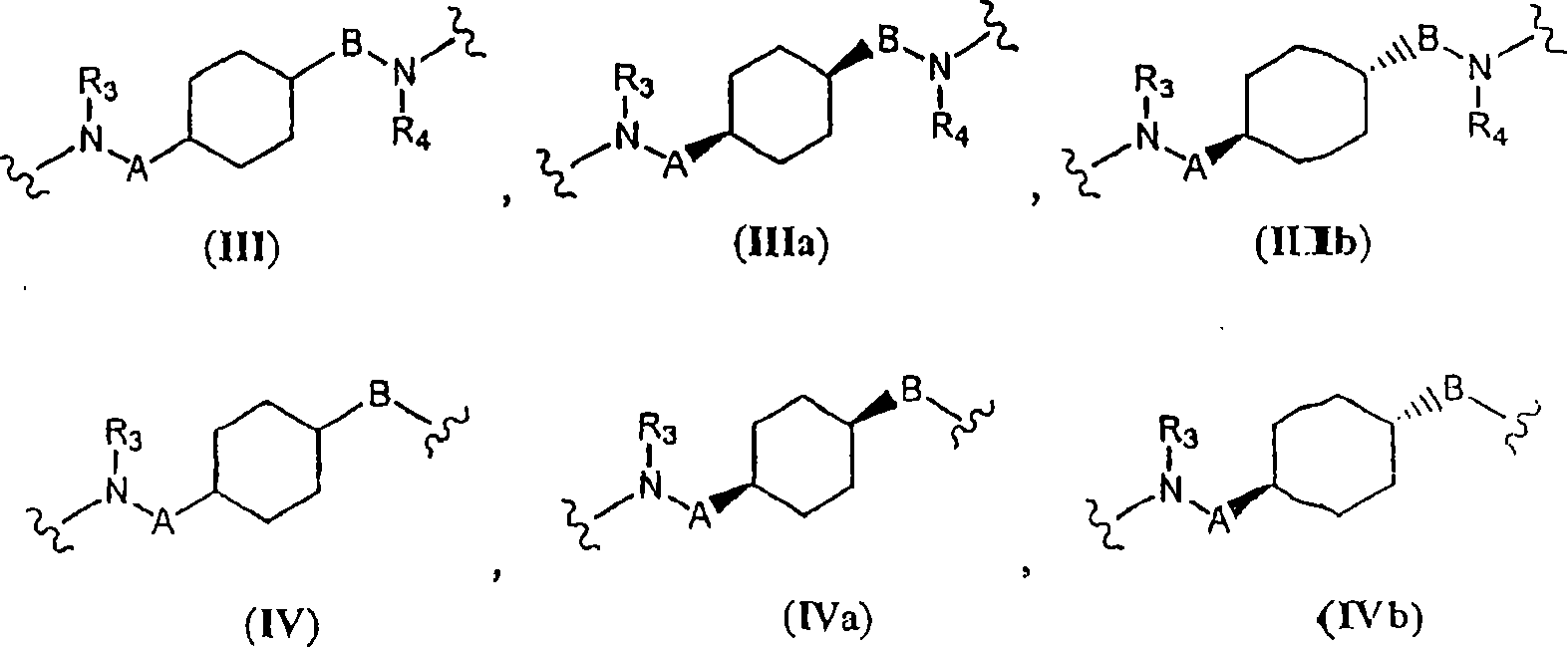
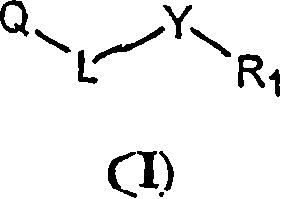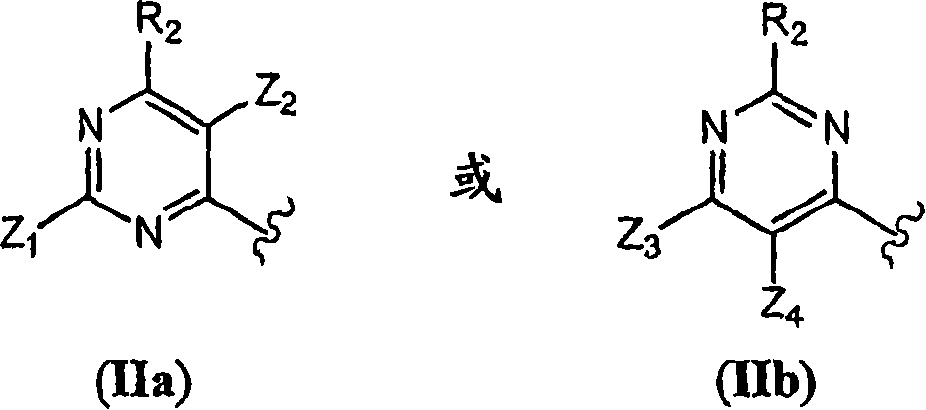Pyrimidine derivatives and methods of treatment related to the use thereof
A technology of compounds and hydrates, applied in organic chemistry, drug combinations, pharmaceutical formulations, etc., can solve problems such as energy intake and consumption imbalance, without considering muscle proportions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1429] Preparation of compound of formula (I)-general synthetic method
[1430] The novel substituted pyrimidines of the present invention can be easily prepared according to various synthetic methods, all of which are familiar to those skilled in the art. Preferred methods for preparing the compounds of the present invention include, but are not limited to those described in Schemes 1-8.
[1431] Pyrimidine (C) can be prepared as shown in Scheme 1. With or without alkali, 4,6-dihydroxypyrimidine (A), which is commercially available or obtained by condensation of malonic acid derivatives and amidine derivatives, can be obtained by halogenating agents, wherein Z 1 And Z 2 As defined above, it is converted to 4,6-dihalo-pyrimidine (B) (where X is halogen, such as chlorine, bromine or iodine). The halogenating agent includes phosphoryl chloride (POCl 3 ), phosphoryl bromide (POBr 3 ) Or phosphorus pentachloride (PCl 5 ). The base includes tertiary amines (preferably N,N-diisopropylet...
Embodiment 1
[1540] N'-(cis-4-{[4-bromo-2-trifluoromethoxy)benzyl]amino}cyclohexyl)-N,N-dimethylpyrimidine-4,6-diamine dihydrochloride
[1541] Step A: (6-Chloro-pyrimidin-4-yl)-dimethyl-amine synthesis
[1542] Add iPr to a solution of 4,6-dichloro-pyrimidine (10.0g) in THF (10mL) 2 NEt (10.4g) and 50% Me 2 NH aqueous solution (6.05 g). After stirring the resulting mixture at room temperature for 28 hours, pour into saturated NaHCO 3 In aqueous solution. CHCl for water layer 3 Extraction (three times). MgSO for combined organic layer 4 Dry, filter, and concentrate under reduced pressure. Suspend the residue in Et 2 O in. Filter to collect the precipitate, Et 2 Wash with O and dry under reduced pressure to obtain (6-chloro-pyrimidin-4-yl)-dimethyl-amine (6.37 g).
[1543] ESI MS m / e 157, M + ; 1 H NMR(300MHz, CDCl 3 )δ3.12(s, 6H), 6.41(s, 1H), 8.37(s, 1H).
[1544] Step B: Synthesis of N-(cis-4-bromo-2-trifluoromethoxy-benzyl)-cyclohexane-1,4-diamine
[1545] To (4-amino-cyclohexyl)-carbamic a...
Embodiment 2
[1551] N-(cis-4-{[6-(dimethylamino)pyrimidin-4-yl]amino}cyclohexyl)-3,4-difluorobenzamide hydrochloride
[1552] Step A: Synthesis of (cis-4-{[1-(3,4-difluoro-phenyl)-formyloxy]-amino}-cyclohexyl)-carbamic acid tert-butyl ester
[1553] To the DMF solution (50 mL) of 3,4-difluoro-benzoic acid (4.10g) and (cis-4-amino-cyclohexyl)-carbamic acid tert-butyl ester (5.05g) was added Et 3 N(90mL), HOBt-H 2 O (5.41g) and EDC-HCl (4.97g). The resulting mixture was stirred at room temperature for 17 hours. Water (200 mL) was added to the reaction mixture, and the resulting suspension was stirred at room temperature for 10 minutes. Filter to collect the precipitate, use H 2 O and EtOH washed, dried under reduced pressure at 80 ℃ to obtain (cis-4-{[1-(3,4-difluoro-phenyl)-formyloxy]-amino}-cyclohexyl)-carbamic acid tert-butyl Esters (5.20 g).
[1554] ESI MS m / e 377, M+Na + ; 1 H NMR(300MHz, CDCl 3 )δ1.45 (s, 9H), 1.53-1.95 (m, 8H), 3.60-3.74 (m, 1H), 4.00-4.16 (m, 1H), 4.50-4.68 (m, 1H), 5.95-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com