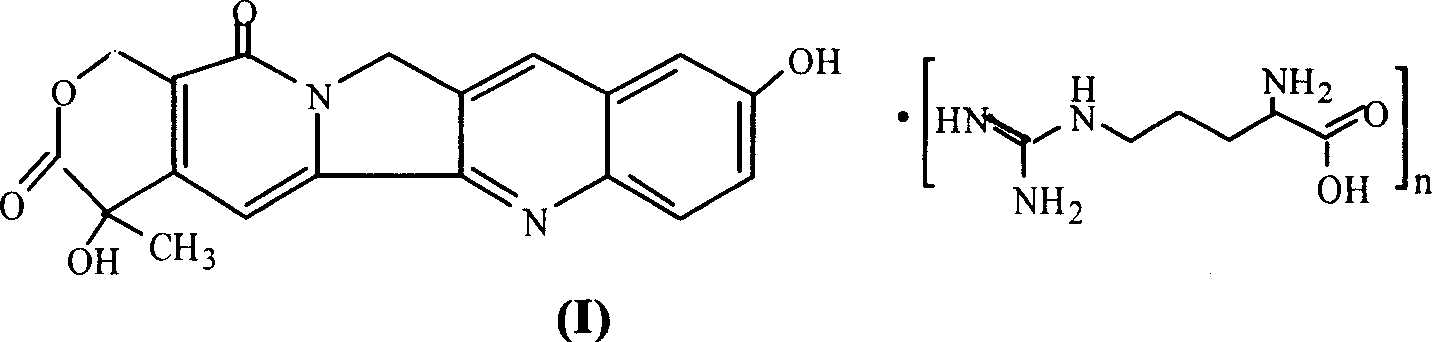
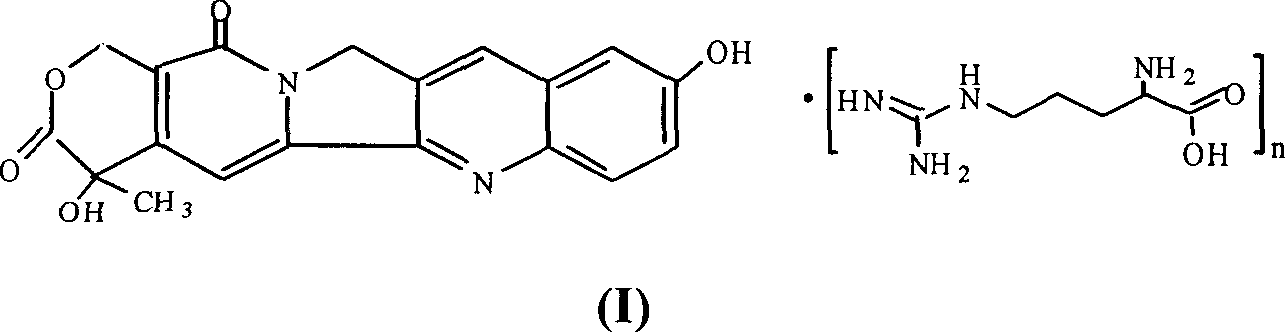
10-hydroxy camptothecin arginine salt, and its preparing method and use
A technology of hydroxycamptothecin and arginine salt, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve problems such as poor solubility, discoloration of solutions, and restricted use, and achieve tumor suppression. Strong activity, strong anti-tumor activity, and safe clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
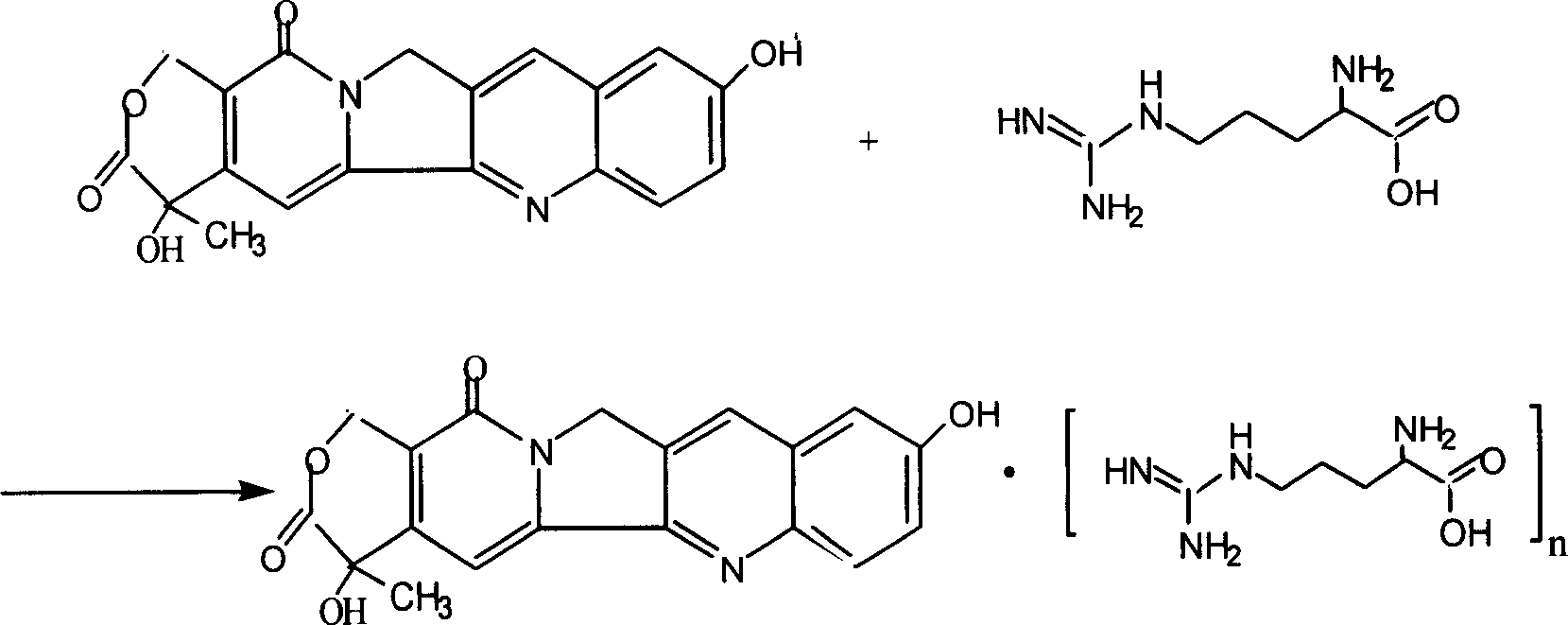
[0025] Embodiment 1 Preparation of 10-hydroxycamptothecin arginine salt (n=1) of the present invention
[0026] Take 728 mg of 10-hydroxycamptothecin and add 100 ml of dimethyl sulfoxide, heat in a water bath to dissolve, add 348 mg of L-arginine under reflux and stirring, continue to reflux until the solution is clear, and recover dimethyl sulfoxide under reduced pressure. Add 25ml of methanol to the dried product, heat to reflux to dissolve, filter, add 250ml of acetone to the filtrate, let it stand, protect from light, filter, and dry the precipitate at 60°C under reduced pressure to obtain a yellow powder precipitate.
[0027] The resulting product has been subjected to the following structural analysis and identification tests:
[0028] Scanning within the wavelength range of 200-500nm, there are maximum absorptions at 210, 266, 367, and 383nm wavelengths, mainly due to the conjugated absorption of hydroxycamptothecin aromatic heterocycles.
[0029] Infrared absorption s...
Embodiment 2
[0042] Embodiment 2 Preparation of 10-hydroxycamptothecin arginine salt (n=6) of the present invention
[0043] Take 240mg and 1.70g of L-arginine respectively, add 100ml of dimethyl sulfoxide, heat in a water bath to dissolve, add 500mg of 10-hydroxycamptothecin under reflux and stirring, continue to reflux until the solution is clear, and recover under reduced pressure Dimethyl sulfoxide, add 25ml of methanol to the dried product and heat to reflux to dissolve, filter, add 250ml of acetone to the filtrate, let stand, protect from light, filter, and dry the precipitate at 60°C under reduced pressure to obtain a yellow powder precipitate. The ninhydrin reaction of this product was positive, and the retention time of HPLC was inconsistent with that of hydroxycamptothecin. The acidic mobile phase was used for analysis, and a peak with a retention time consistent with that of hydroxycamptothecin appeared. There are arginine spots in TLC examination under acidic conditions, that ...
Embodiment 3
[0044] Example 3 Preparation of 10-hydroxycamptothecin arginine salt injection
[0045] R x1 : 100ml: 50mg
[0046] Hydroxycamptothecin arginine salt 0.5g (prepared by embodiment 1)
[0048] 0.1M sodium hydroxide appropriate amount
[0049] Water for injection 1000ml
[0050] Take by weighing the hydroxycamptothecin arginine salt of the prescription amount, add 500ml of water for injection to dissolve, add 8.5g of sodium chloride, stir to dissolve, adjust the pH to 9.2 with 0.1M sodium hydroxide, add 1000ml of water for injection, filter, Ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com