Antiobesity drug
An obesity and amino acid technology, applied in drug combinations, pharmaceutical formulations, growth factors/inducible factors, etc., can solve problems such as ignorance of the physiological function of AGF
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] Hereinafter, although an Example demonstrates this invention concretely, it does not limit the scope of the present invention. In addition, unless otherwise specified, it can implement according to a well-known method ("Molecular Cloning", Sambrook, J etc., Cold Spring Harbor Laboratory Press, 1989 etc.). Also, when using a commercially available reagent or kit, it can be carried out according to the instructions of the commercially available product.
[0105] Regarding the preparation of gene knockout animals and transgenic animals, if there is no special instructions, it can be obtained according to Manipulating the Mouse Embryo.A Laboratory Manual.2nd Edition, B.Hogan, R.Beddington, F.Costantini, E.Lacy, Cold Spring Harbor , New York, Cold Spring Harbor Laboratory Press, 1994 implementation; Regarding the preparation of chimeric mice using ES cells, it can be based on AL Joyner: Gene Targeting, A Practical Approach, OXFORD UNIVERSITY PRESS, 1993 or バイオマニュアルシリズ 8, ジ O...
reference example 1
[0106] "(Reference Example 1: Preparation of AGF KO Mice"
[0107] (1) Preparation of target carrier
[0108] The following method was used to prepare the genome sequence (5' long arm (long arm)) of the 5' side of the mouse AGF gene, the pgk promoter, the neomycin resistance gene, and the exon containing the mouse AGF gene ( A part of exon) 2 and the genomic sequence (3' short arm (shortarm)) of exon 3, and the target carrier of HSV-tk gene.
[0109] That is, cDNA corresponding to the full-length protein coding region of mouse AGF was prepared in the same manner as in Example 1 of WO03 / 083114. The cDNA was used as a probe to screen the mouse genome library (Mouse Genomic, 129 SVJ Library; Stratagene Company) according to the attached instructions, and isolate a sequence with about 17.9 kbp (containing AGF gene, mouse-pub-genome sequence ACO73775.2 90644 ~ 108544) of the phage clone, subcloned in the plasmid pBlueseript (Stratagene Company). The plasmid clone (pBN2) was cut ...
Embodiment 1
[0124] "Example 1: Preparation of CAG-AGF Tg mice"
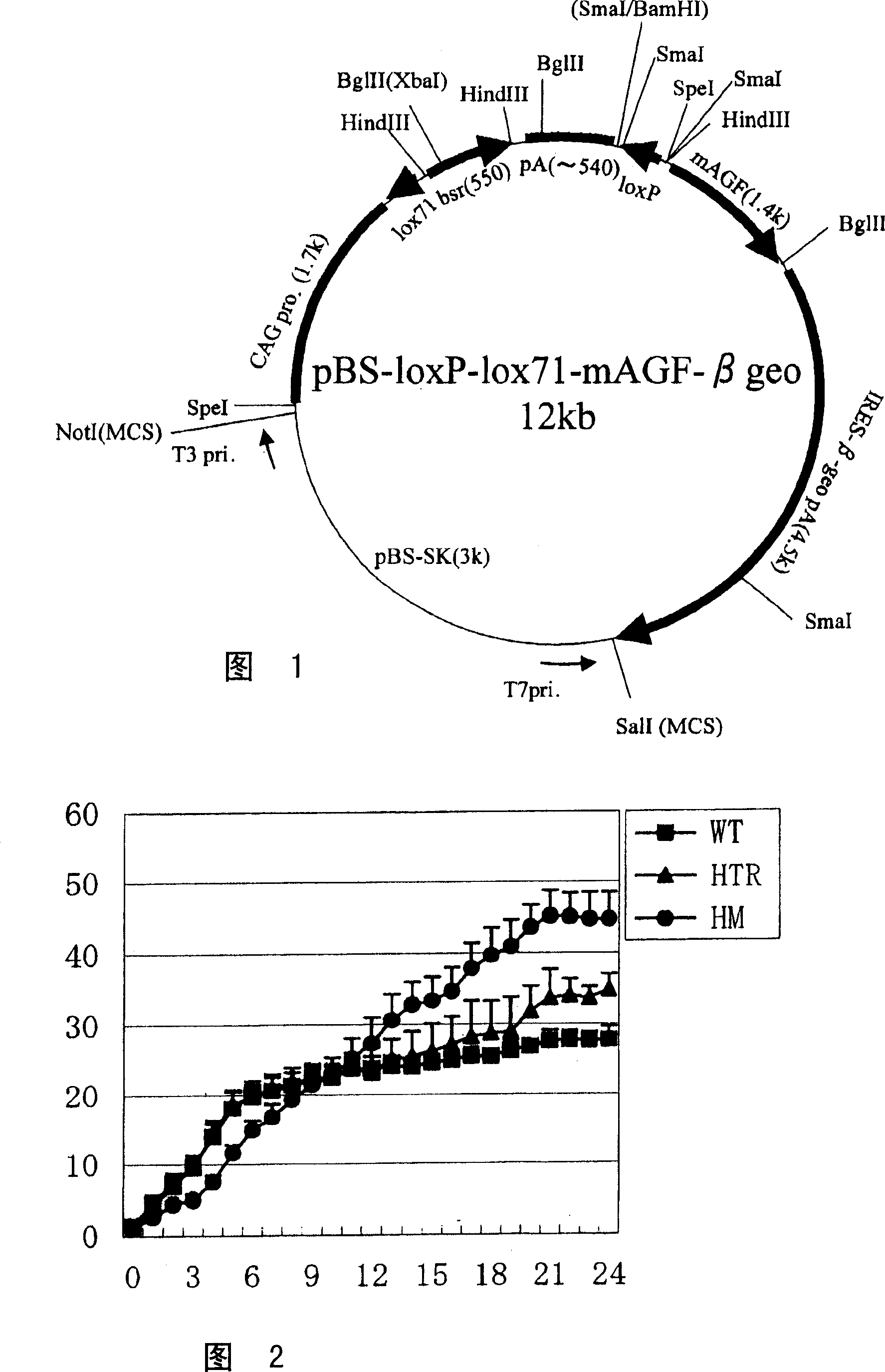
[0125] In this example, AGF transgenic mice (hereinafter referred to as AGF transgenic mice (hereinafter referred to as CAG-AGF Tg mice). Prepare the CAG promoter (1.7kp), lox71 sequence, blasticidin gene (bsr), Poly A signal sequence ( 0.5kb), lox P sequence, mouse AGF cDNA sequence and IRES (internal ribosomal entry site)-β-geo-Poly A sequence (4.5kb) plasmid.
[0126] Mouse AGF full-length cDNA (WO03 / 083114 bulletin) was used as a template, and the DNA composed of the base sequence shown in sequence number 13 (AGAAGCTTCACCATGGGGACCGCCAGGCTAC artificial sequence) and sequence number 14 (CCGTCGACATTAGATCTTCACAAGCGCAAGCCGGGTC artificial sequence) was used as a primer set to perform PCR (95 After reacting at °C for 10 minutes, 45 reaction cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 2 minutes) were performed, and the obtained PCR product was subcloned into pZErO-2 cloning vector (Invitrogen). The obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com