Isoflavone derivative, its production and use as antioxidant
A technology of isoflavones and derivatives, applied in the field of new water-soluble antioxidants, can solve the problems of poor water-solubility and fat-solubility, slow absorption, troublesome extraction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
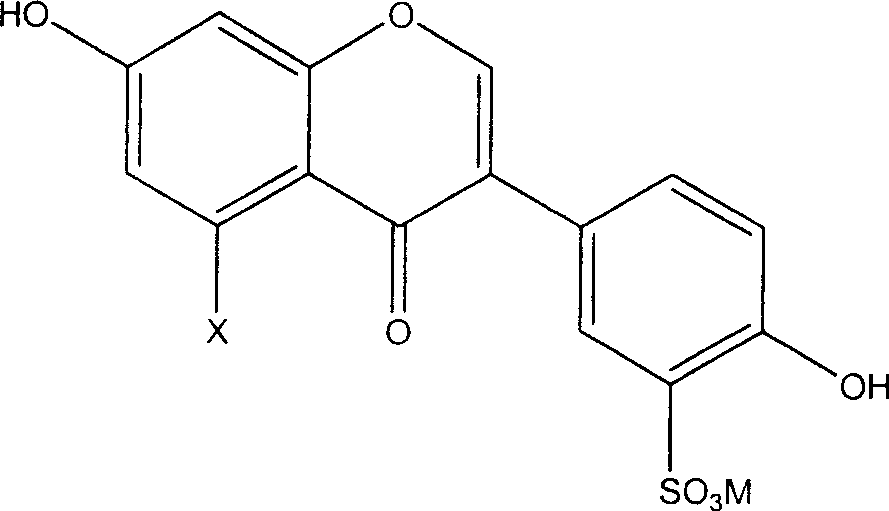
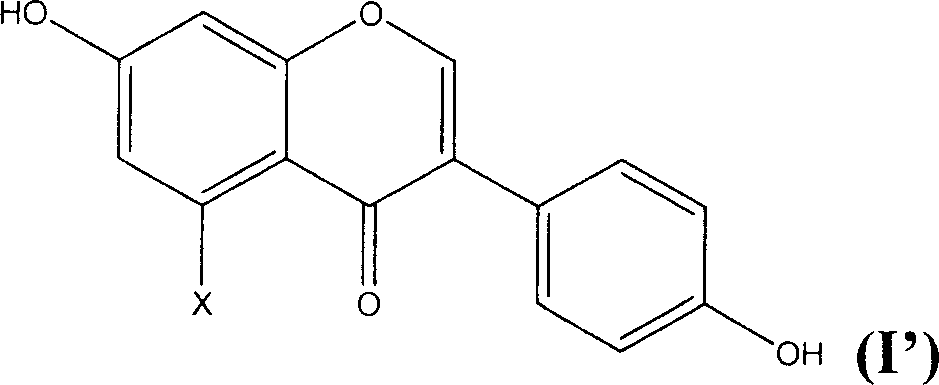
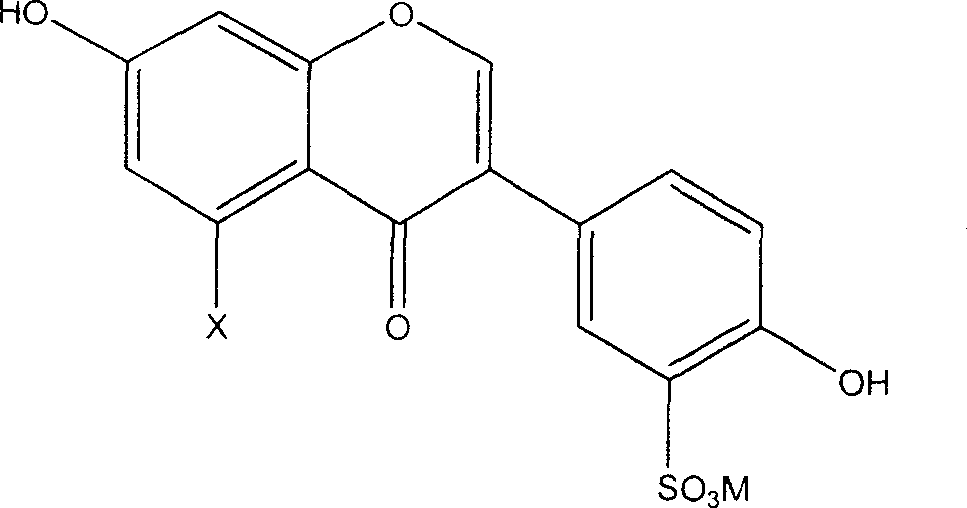
[0018] The preparation method of the novel antioxidant of the present invention comprises sulfonating the general formula (I') and using an alkali metal or alkaline earth metal compound to form a salt, thereby introducing a sulfonate group to obtain an antioxidant with better water solubility:
[0019]
[0020] Wherein X is hydrogen or hydroxyl.
[0021] More specifically, when X in the above general formula (I') is hydrogen, the reaction is as follows:
[0022]
[0023] wherein M is as defined above.
[0024] When X in the above general formula is a hydroxyl group, the reaction is as follows:
[0025]
[0026] wherein M is as defined above.
[0027] Specifically, the preparation of 3'-sulfonic acid group-daidzein metal salt (L3) and 3'-sulfonic acid group-genistein metal salt (L4) can be carried out as follows: make daidzein (L1 ) or genistein (L2) reacts with concentrated sulfuric acid and adds an alkali metal or alkaline earth metal compound to make the sulfo gr...
Embodiment 1
[0031] Synthesis of Antioxidant L3
[0032] Take 2.52g (0.01mol) L1 (supplied by Shaanxi Saide Hi-Tech Biological Co., Ltd.) into 10mL of concentrated sulfuric acid, stir at room temperature for 30 minutes, the solid gradually dissolves, and the solution turns dark yellow. Take a small amount of reaction liquid and drop it in water, if no insoluble matter appears, it means that the reaction is complete. The reaction was slowly and carefully poured into 40 mL of ice water and stirred. Solid NaCl was then added to the filtrate and stirred vigorously until the added NaCl was no longer dissolved. Stand still to form a white flocculent precipitate, filter and wash with saturated saline. Recrystallized with 5% NaCl aqueous solution to obtain 2.6 g of white needle-like crystal daidzein-3'-sulfonic acid sodium salt with a yield of 75%. m.p.349℃, IR(KBr, ν, cm -1 ): 3441.4(-OH), 1635(-CO-), 1193(-SO 3 ); 1 H NMR (D 2 O, 500MHz, δ, ppm): 6.59 (s, 1H, HC-6), 6.64 (s, 1H, HC-8), ...
Embodiment 2
[0034] Synthesis of Antioxidant L4
[0035] Take 2.69g (10mmol) L2 (supplied by Shaanxi Saide Hi-Tech Biological Co., Ltd.) into 10mL concentrated sulfuric acid, stir at room temperature for 30 minutes, the solid gradually dissolves, and the solution turns dark yellow. Take a small amount of reaction liquid and drop it in water, if no insoluble matter appears, it means that the reaction is complete. The reaction was slowly and carefully poured into 40 mL of ice water and stirred. Solid NaCl was then added to the filtrate and stirred vigorously until the added NaCl was no longer dissolved. Stand still to form a white flocculent precipitate, filter and wash with saturated saline. Recrystallized with 5% NaCl aqueous solution to obtain 2.8 g of white needle crystals (L4) (yield 78%). m.p.>260℃(decomposition), IR(KBr, ν, cm -1 ): 3441.4(-OH), 1642.5(-CO-); 1 H NMR (D 2O, 500MHz, δ, ppm): 5.89 or 5.91 (s, 1H, HC-6 or HC-8), 6.90 or 7.02 (d, 1H, HC-5' or HC-6'), 7.59 (s, 1H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com