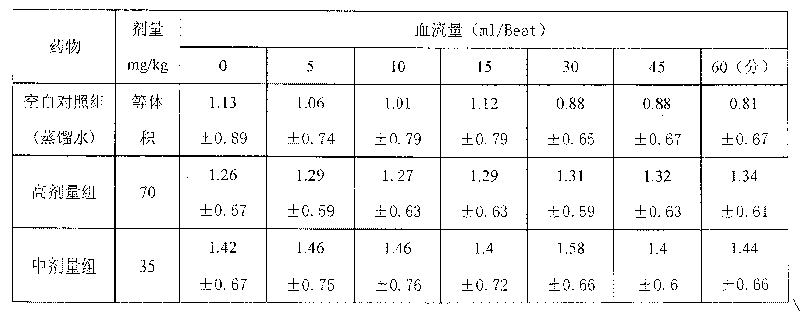
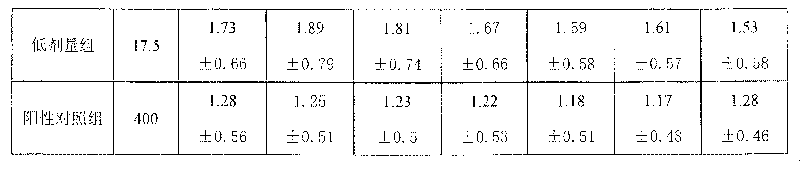
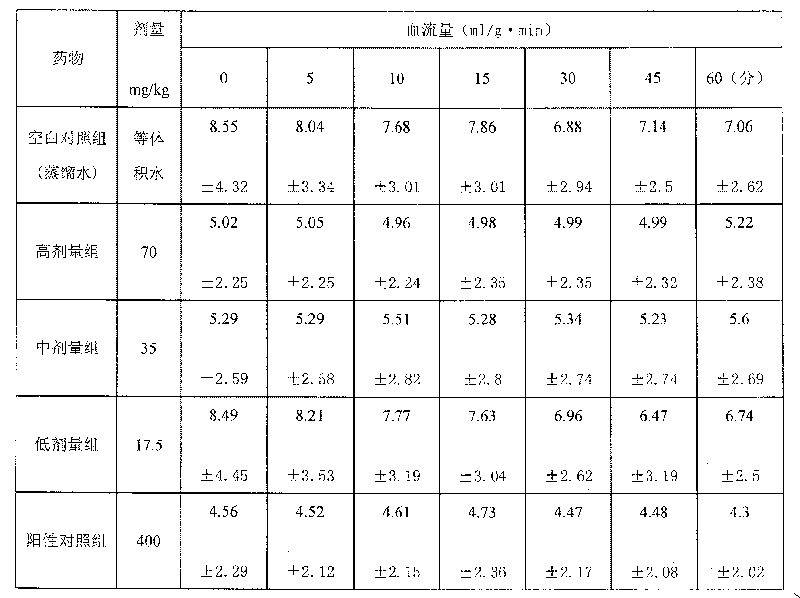Medicinal composition for treating cardio-cerebral vascular disease and its preparing method
A technology for cardiovascular and cerebrovascular diseases and compositions, which is applied in the field of pharmaceutical compositions for the treatment of cardiovascular and cerebrovascular diseases and its preparation. The effect of enhancing cerebral blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] Monomer preparation:
[0019] Extract Pueraria lobata with water or dilute alcohol, concentrate the obtained extract, perform silica gel column chromatography, chloroform-methanol gradient elution, and combine the fractions according to TLC chromatography, and then purify with gel Sephadex LH-20 column to obtain The physical and chemical properties of the following components are as follows:
[0020] 1. Formononetin-7-O-glucoside: molecular formula C 22 h 22 o 9 , white crystals, mp.210-2°C.
[0021] UV lambda max (nm) 256,300 (shoulder).
[0022] 1 HNMRδppm (500MHz, DMSO-d 6 Middle, the same below), 8.38 (1H, s, H-2), 8.04 (1H, d, J=9.0Hz; H-5), 7.14 (1H, d, J=9.0Hz, H-6), 7.21 (1H, s, H-8), 7.50 (2H, d, J=9.0Hz, H-2′, 6′), 6.97 (2H, d, J=9.0Hz, H-3′, 5′), 5.12 (1H, d, J = 7.0Hz, sugar terminal proton), 3.78 (3H, s, OCH 3 ).
[0023] 13 CNMRδppm (125MHz, DMSO-d 6 Middle, the same below), 153.5(C-2), 123.9(C-3), 174.5(C-4), 126.8(C-5), 115.5(C-6), 161.4(C-7...
Embodiment 1
[0069] The preparation of embodiment 1 capsule
[0070] Weigh each component according to the following parts by weight: 5 parts of formononetin-7-O-glucoside, 7 parts of daidzein, 5 parts of 3′-methoxyglycoside puerarin, 49 parts of puerarin, and 2 parts of puerarin B , 19 parts of daidzein-8-C-apirosyl-(1-6)-glucoside, 3 parts of puerarin xyloside, 1 part of daidzein-7,4'-diglucoside, puerarin-4' 3.4 parts of -0-glucoside, 0.4 parts of mephrasin-4'-glucoside, 1 part of 4'-methoxygenistin, 5 parts of 3'-hydroxypuerarin, and 1 part of puerarin C; After the components are uniformly mixed, add (or not add) excipients such as dextrin, starch, sucrose, etc., granulate, dry, mix, and fill capsules to obtain.
Embodiment 2
[0071] The preparation of embodiment 2 oral liquid
[0072] Weigh each component according to the following parts by weight: 4.5 parts of formononetin-7-O-glucoside, 6.7 parts of daidzein, 4.5 parts of 3′-methoxypuerarin, 44 parts of puerarin, and 1.8 parts of puerarin B , 17 parts of daidzein-8-apigenyl-(1-6)-glucoside, 2.7 parts of puerarin xylose sprouts, 0.9 parts of daidzein-7,4'-diglucoside, 3'-hydroxypuerarin 4.5 parts, 3 parts of puerarin-4'-O-glucoside, 0.4 part of mefosine-4'-glucoside, 0.9 part of 4'-methoxygenistin, 0.9 part of puerarin C; Dissolve in sterilized distilled water after mixing evenly, add syrup, preservatives and other auxiliary materials, constant volume, filter, fill, sterilize, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com