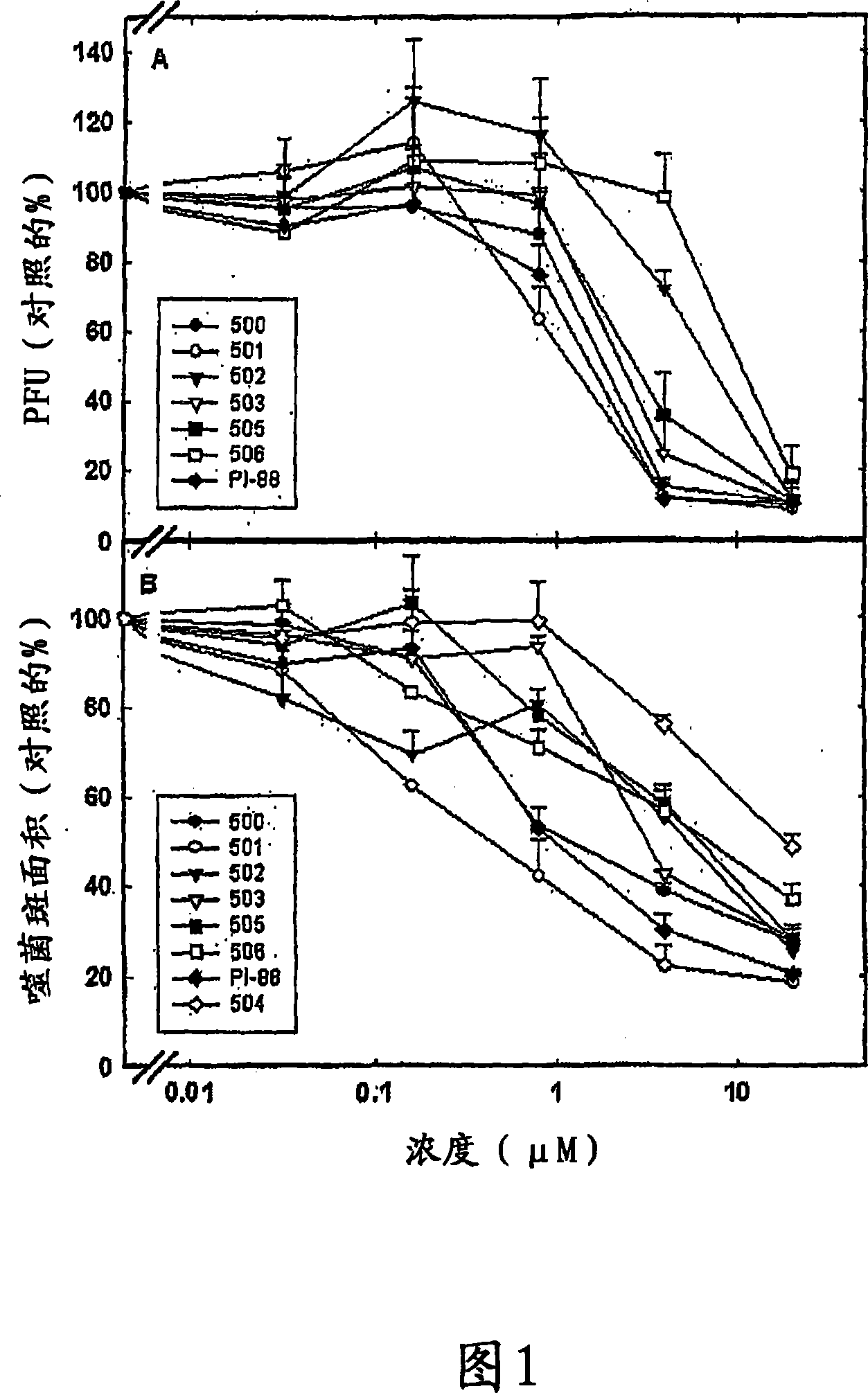
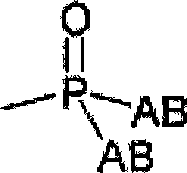
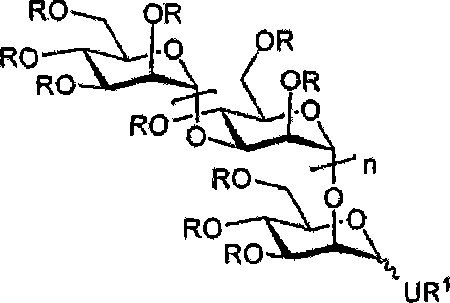
Sulfated oligosaccharide derivatives
A kind of technology of derivatives and compounds, applied in the field of sulfated oligosaccharide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Total Synthesis of Neutral Manno-Oligosaccharides from Pichia (8-11)
[0086]
[0087] Benzyl 2-O-(3-O-allyl-2,4,6-tri-O-benzoyl-α-D-mannopyranosyl)-3,4,6-tri-O- Benzyl-α-D-mannopyranoside (24)
[0088] 3-O-allyl-2,4,6-tri-O-benzoyl-α-D-mannopyranosyl trichloroimidate [26] (902mg, 1.21mmol) and benzyl A mixture of 3,4,6-tri-O-benzyl-α-D-mannopyranoside [27] (723mg, 1.34mmol) in 1,2-DCE (10mL) over molecular sieves (1.0g of 3 - powder) under argon atmosphere (30 min). The mixture was cooled (0° C.) with continued stirring (10 minutes) before adding TMSOTf (219 μL, 1.21 mmol). After a period of time (10 minutes), Et was introduced3 N (100 μL) and the mixture was filtered. The solvent was evaporated and the residue was subjected to FC (10-50% EtOAc / hexanes) to give the tribenzoate (24) (1.14 g, 84%) as a colorless oil. 1 H NMR (CDCl 3 )δ3.67-3.81, 3.88-3.95, 4.06-4.15, 4.30-4.35 (4m, 12H; H-2 I , -3 I , -4 I , -5 I ,-6a I ,-6b I , -3 II , -5 II ...
Embodiment 2
[0110] Embodiment 2: benzyl glycoside polysulfate (PG 500)
[0111]
[0112] Peracetate 12
[0113] The pentasaccharide 11 (1.03 g, 95% M5), sodium acetate (1.2 g) and acetic anhydride (50 mL) were heated under a drying tube at 140° C. overnight with stirring. The mixture was cooled to room temperature, evaporated to dryness, taken up in EtOAc, washed with brine (x 3) and flash chromatographed (40 g silica gel, 80:20 EtOAc:Hx) to give a glassy form with very little pure material. 810mg of peracetate 12. 1 H NMR (400MHz, CDCl 3 )δ6.14(d, 0.84H, J=2.0, αH1 I ), 5.71 (d, 0.16H, J=0.9, βH1 I ), 5.30-5.10 (m, 8H), 5.00-4.85 (m, 7H), 4.25-3.70 (m, 19H), 2.20-1.90 (m, 51H). for C 64 h 87 o 43 Calculated value of HRMS [M+H] + 1543.4623, found 1543.4599.
[0114] General method for direct saccharification of peracetylated oligosaccharides:
[0115] The alcohol (6 equiv) was added to a solution of the peracetate (eg, 12) (1 equiv) in 3-MS dry DCM (0.03M). In some cases, a s...
Embodiment 3
[0122] Embodiment 3: Octyl glucoside polysulfate (PG 501)
[0123]
[0124] Octyl Glycoside 14
[0125] The saccharification using 12 and octanol afforded the product (14) as a colorless gum, 207 mg, 66% (Rf = 0.41, hexane-EtOAc = 1 :3). 1 H NMR (CDCl 3 , 400MHz) δ5.23-5.09(m, 8H), 4.96-4.82(m, 8H), 4.23-3.71(m, 19H), 3.59(dt, 1H, J=9.4, 6.8, OCH 2 R), 3.35 (dt, 1H, J=9.4, 6.8, OCH 2 R), 2.11, 2.10(2), 2.09(8), 2.06, 2.05, 2.04(4), 2.04(1), 2.03(8), 2.03, 2.02, 2.01, 1.99(3), 1.98(8), 1.96, 1.94 and 1.90 (16s, 48H, 16×Ac), 1.52 (quintet, 2H, J=7.2, CH 2 ), 1.27-1.18 (m, 10H, (CH 2 ) 5 ), 0.80(t, 3H, J=7.2, CH 3 ); 13 C NMR (CDCl 3 , 100MHz) δ170.4(0)(2C), 170.3(8)(2C), 170.3, 170.2, 170.1, 169.9(2C), 169.8(2), 169.7(5), 169.6, 169.5, 169.4(4) , 169.3(5), 169.3 (16×CO, 3 overlapping), 99.1(2C), 98.8, 98.7, 98.0 (5×sugar-C1), 77.0, 75.0, 74.8(3), 74.7(5), 71.0 , 70.8, 70.7, 70.1, 69.4(9), 69.4(7), 69.3(0), 69.2(7), 69.2, 68.3, 68.2(0), 68.1(6), 67.2, 66.6(4), 66.6(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com