Liver function test
A technology of liver function and bile acid derivatives, which can be used in biological testing, testing pharmaceutical preparations, immunoassays, etc., and can solve problems such as expensive and complicated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
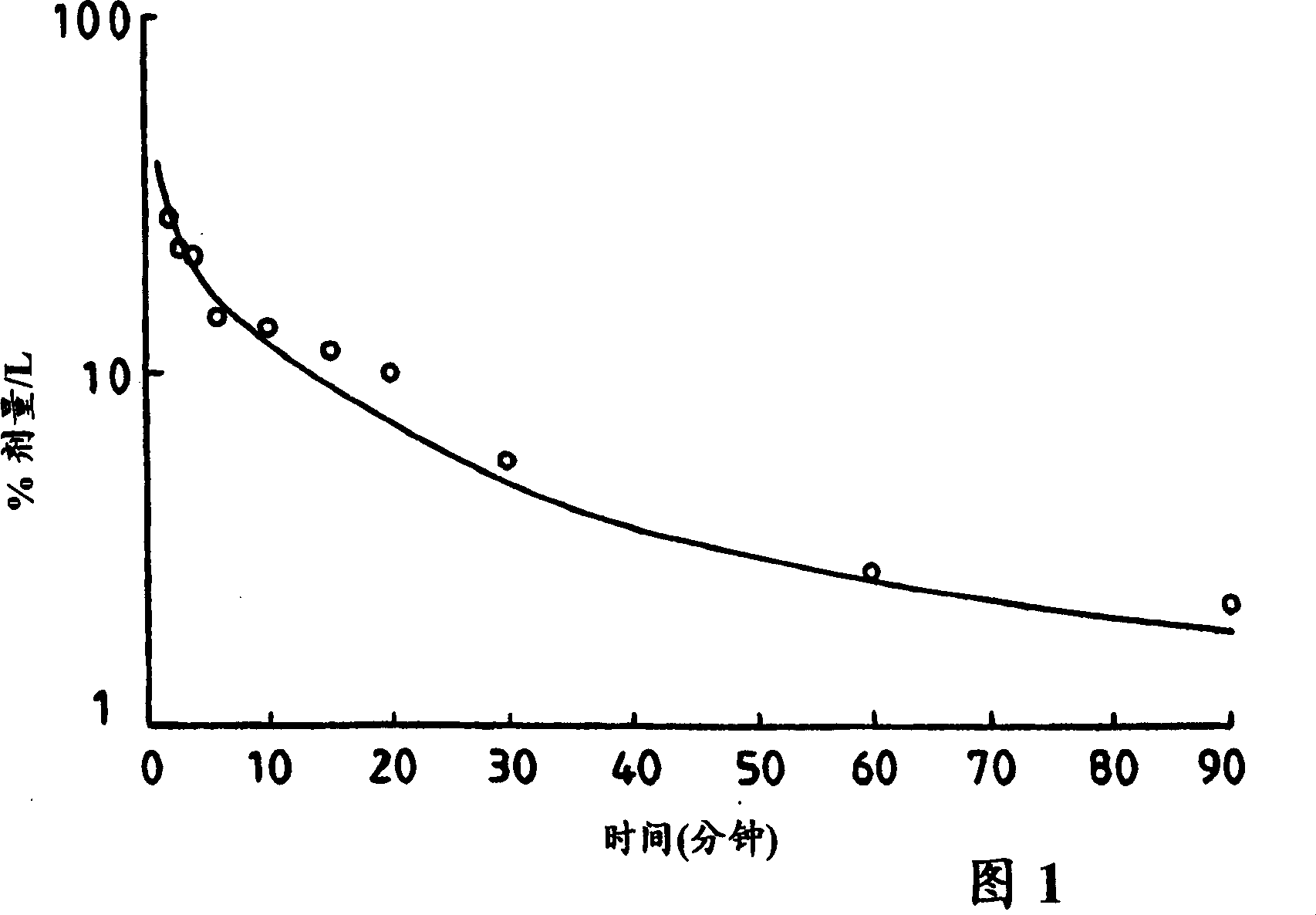
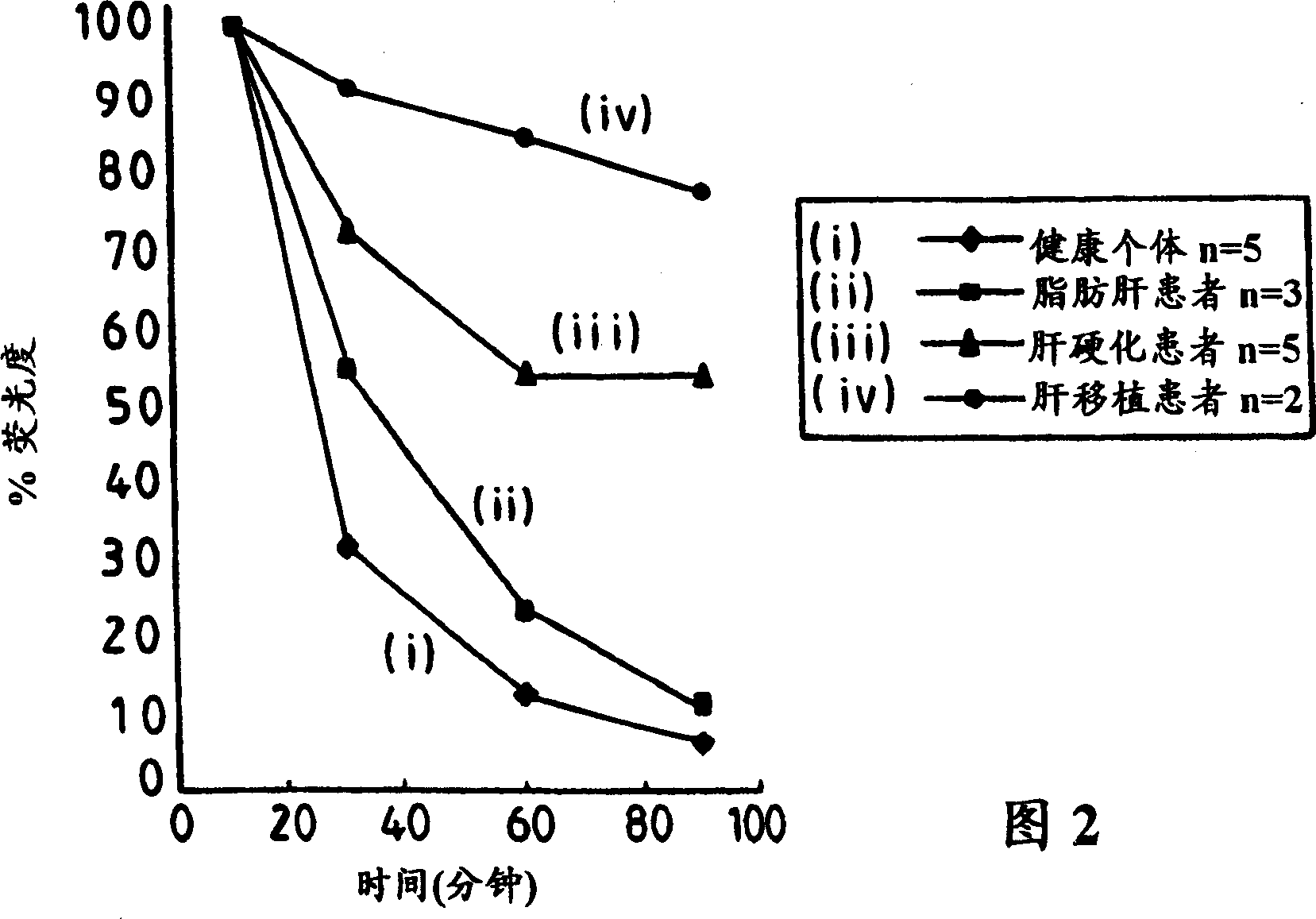
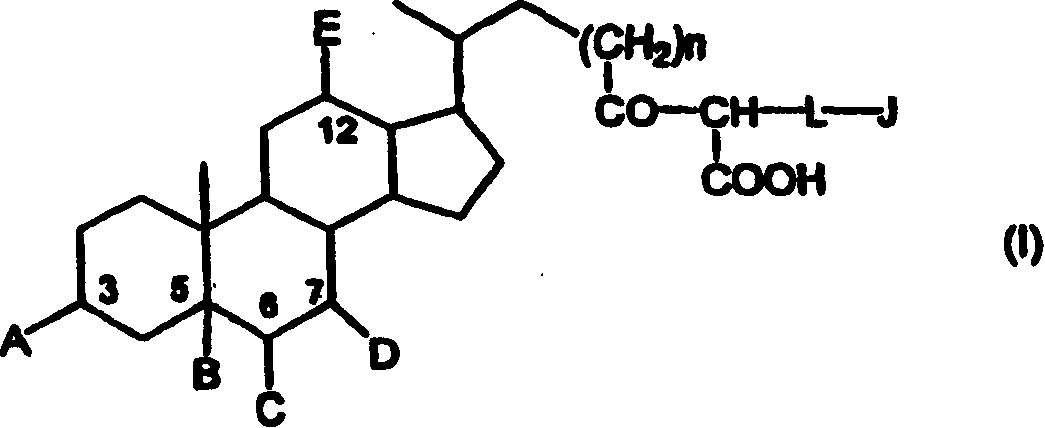
[0053] CLF was synthesized according to the method described elsewhere [Mills et al.'s "Biochemistry and Biophysics Acta", (1991) 1115, 151], sterilized and tested for the presence of heat Original.
[0054] The study was carried out with 6 healthy volunteers (1 female, 5 males) aged between 30-53 years. Subjects were placed supine after an overnight fast. After the initial basal blood sample was taken, CLF was injected intravenously at a dose of 0.02 mg / kg body weight (b.w.) in saline over 15 seconds. At 2, 3, 4, 6, 10, 15, 20, 30, 60, and 90 minutes, venous blood samples were collected from an antecubital facing vein and placed in gel and lithium heparin containers (trade name Vacutainers, manufactured by Becton Dickinson Vacutainer Systems). The blood samples were then centrifuged and a 0.5 ml aliquot of plasma was removed and added to 3.5 ml methanol to precipitate plasma proteins, followed by centrifugation again. The supernatant (1 ml) was diluted to 3 ml with methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com