Chelating polymeric membranes
a polymer membrane and polymer technology, applied in membranes, membrane technology, separation processes, etc., can solve the problems of large amount of organic solvents, and/or irreversible adsorption of metals,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Polythiosemicarbazide (PTSC) Synthesis
[0063]Instructions for synthesizing the PTSC shown in Formula III can be found in Campbell and Tomic, “Polythiosemicarbazides 1. Preparation and Properties of Polymers and Some Simple Metallic Chelates,” J. Polymer Science, 62(174): 379-386 (1962), which is hereby incorporated by reference in its entirety.
[0064]For example, the PTSC shown in Formula III was made by stirring a solution containing 3.1 wt % 1,4-diaminopiperazine, 7.6 wt % of 4,4-methylenebis (phenyl isothiocyanate) and 89.2 wt % of DMSO at 50° C. for 24 hours. The resulting polymer was precipitated in water, chopped into small pieces, washed again with water and dried
example 2
Microporous PTSC Film
[0065]A PTSC polymer was prepared in accordance with Example 1. A polymer solution was prepared comprising 15 wt % PTSC, 75 wt % DMSO, and 10 wt % 1,4-dioxane by mixing the 3 components and stirring for approximately 5 hours at room temperature. The resulting viscous liquid rested for 5 hours to permit the escape of any air bubbles.
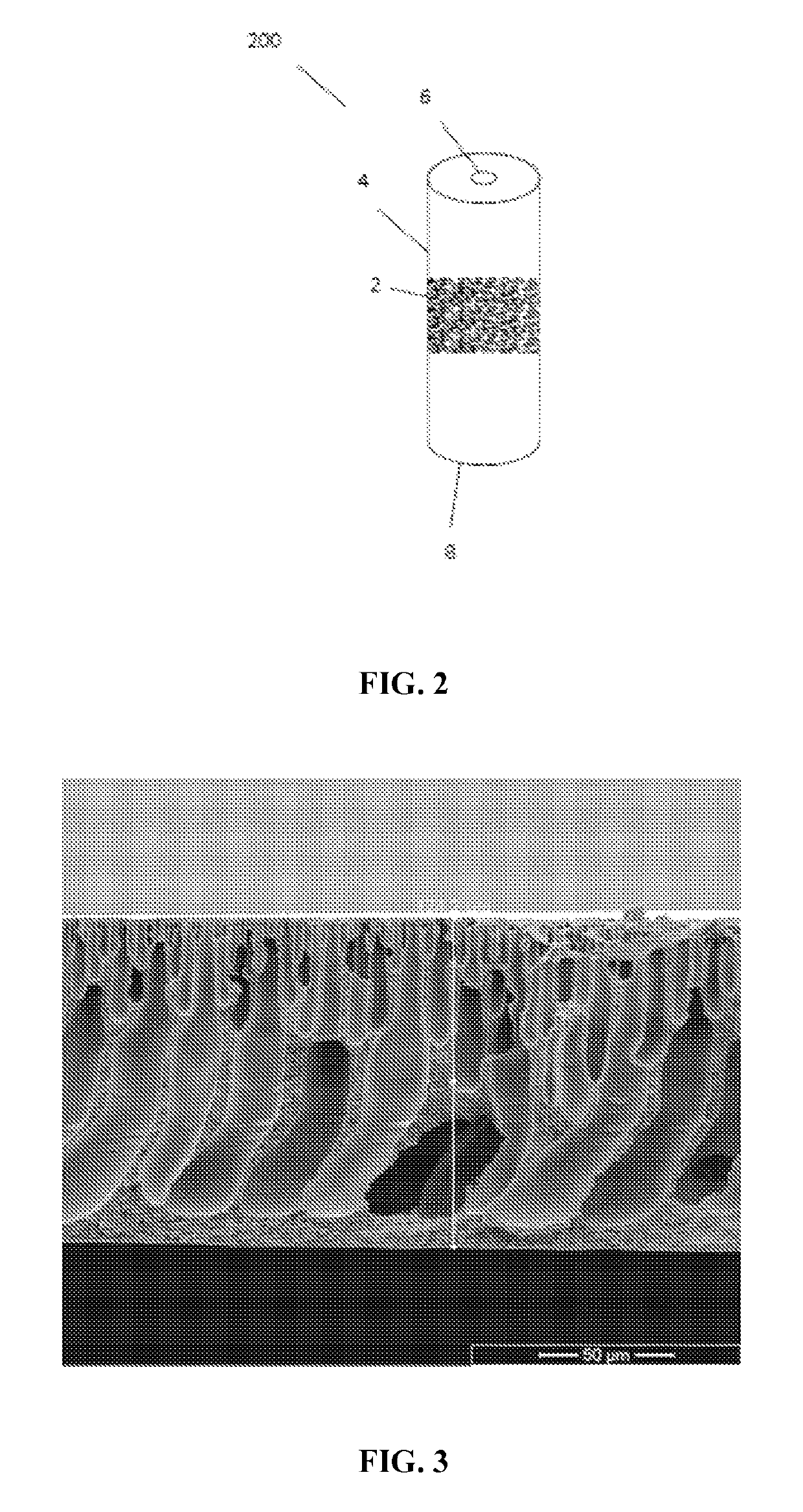
[0066]The polymer solution was casted onto a glass plate into a 250 μm thick film with the use of a doctor knife. The glass plate was then immersed into a non-solvent bath (tap water) at room temperature for at least 12 hours. The membrane was stored in a water bath until use. FIG. 3 depicts a cross-section of the resulting asymmetric membrane.
example 3
Gold Recovery with Microporous PTSC Membrane at Flux=101 L / M2H
[0067]A microporous membrane prepared in accordance with Example 2 was tested with the method(s) described below to assess gold loading of the membrane. The membrane was cut into a four circles, each with an approximate diameter of 2.5 cm and an average dry weight of 14.3 mg. Each membrane was placed on a non-woven polyester circular support of approximately the same size.
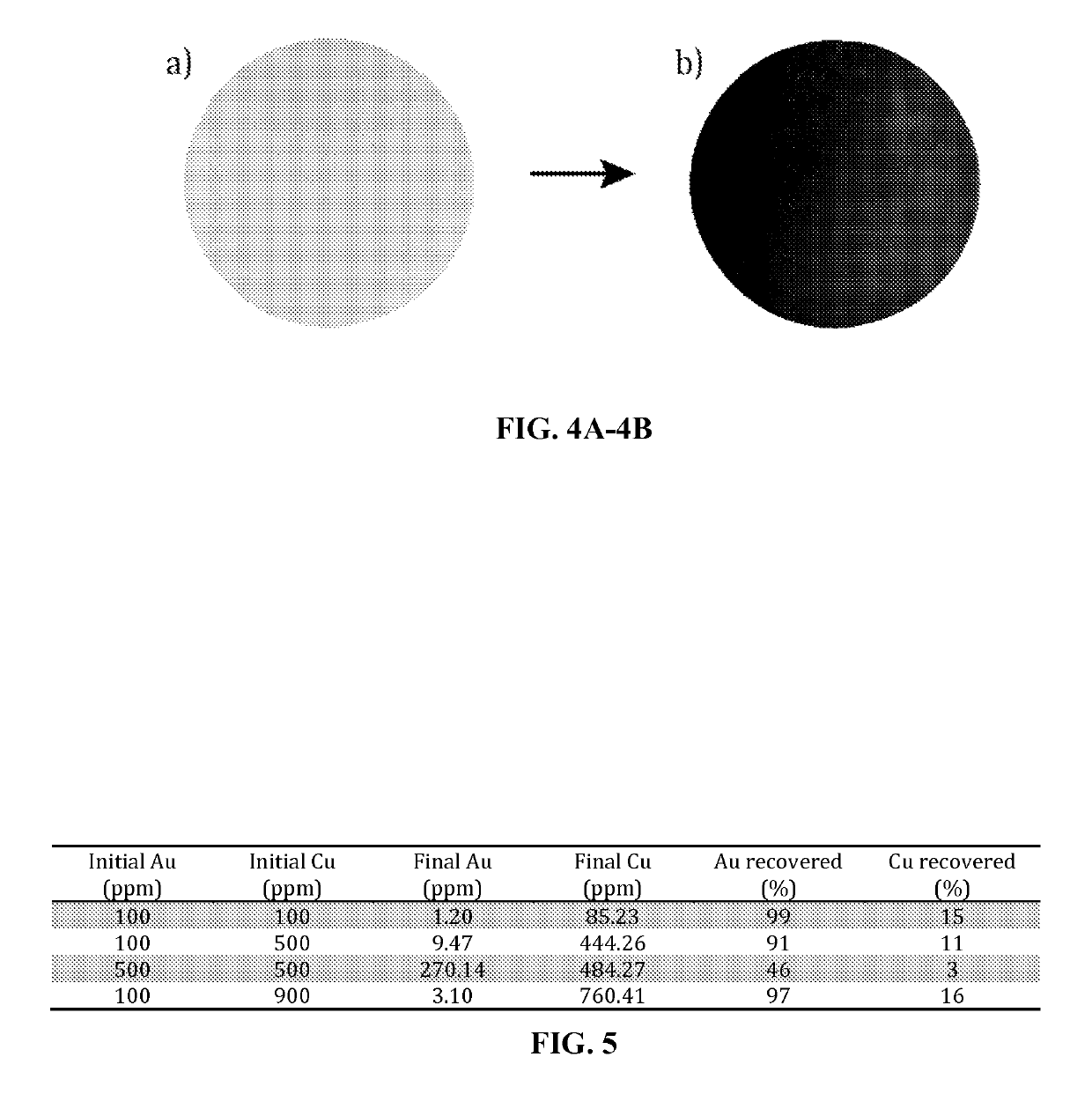
[0068]Four aqueous solutions containing gold in 10% HCl were prepared at 100 ppm, 200 ppm, 500 ppm, and 1000 ppm. 10 mL of each solution permeated through a membrane with the use of pressurized air. A difference in pressure of 0.5 bar was sufficient to permeate the solutions through the respective membrane with a flux of 101 L / m2h. FIGS. 4A and 4B depict the membranes before gold absorption (4A) and after gold absorption (4B). Prior to gold absorption, the membrane is an off-white color, and afterward, the membrane is a brownish color. The amount of gold...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com