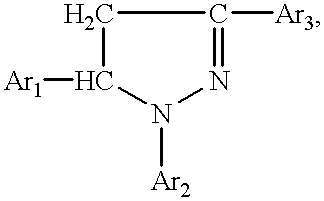
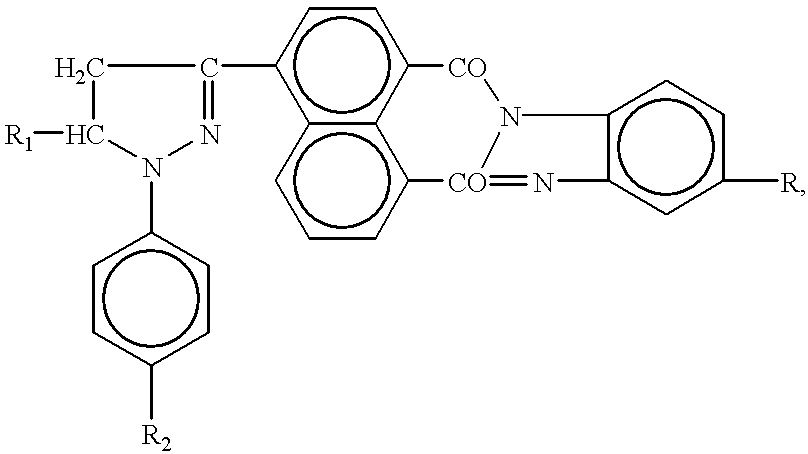
Synthesis of pyrazolinylnaphthalic acid derivatives
a technology of pyrazolinylnaphthalic acid and derivatives, which is applied in the field of synthesis of pyrazolinylnaphthalic acid derivatives, can solve the problems of increasing production costs, low product containing considerable amounts of impurities, so as to enhance the yield and quality of final products, simplify the process, and reduce the time it takes to synthesize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of 4-(1,5-diphenyl-2-pyrasolinyl-3)-N-phenylnaphtalimide
[0126] A mixture of 10.8 g of 4-acetylnaphtalic anhydride (obtained by the acetylation of acenaphtene with acetic anhydride in the presence of anhydrous zinc chloride and by the oxidation of the formed acetylnaphtene with sodium dichromate), 5.3 g of benzaldehyde, and 120 ml of 4.4% caustic soda solution is stirred at room temperature for 3 hours. The precipitate--disodium salt of 4-cinnamoylnaphtalic acid--is separated by filtering and washed with alcohol.
[0127] A mixture of 15.79 g of disodium salt of 4-cinnamoylnaphtalic acid, 4.89 g of aniline and 60 ml of acetic acid is boiled for 6 hours. The reaction mixture is then allowed to cool down to room temperature (20.degree. C.). The formed precipitate is separated by filtering and washed on the filter with water and hot alcohol.
[0128] A mixture of 12.1 g of 4-cinnamoyl-N-naphtalimide, 4.26 g of phenylhydrazine, 100 ml of ethyl alcohol and 6 ml of 10% alkaline soluti...
example 2
Production of 4-[1-phenyl-5-(4-methoxyphenyl)-2-pyrazolinyl-3)-N-phenylnap-htalimide
[0130] This compound is produced and cleaned as the product in example 1. The source products are 4-acetylnaphalic anhydride (10.8 g), anisic aldehyde (6.12 g), aniline (4.89 g), and phenylhydrazine (4.1 g).
[0131] The end product is made up of dark red crystals; it is soluble in common organic solvents and water-insoluble. The melting temperature is 215.degree. C. The yield is 12.13 g (80%).
[0132] .sub.max. lum. in toluene is 575 nm.
example 3
Production of 4-[1,5-diphenyl-2-pyrazolinyl-3)-N-(4-methoxyphenyl)]-naphta-limide
[0133] This compound is produced and cleaned as the product in example 1. The source products are 4-acetylnaphalic anhydride (21.6 g), benzaldehyde (9.54 g), n-anisidine (13 g), and phenylhydrazine (10.3 g).
[0134] The end product yield from 31.54 g of the corresponding unsaturated ketone is 31.6 g (83%).
[0135] The formed red crystals have a melting temperature of 268.degree. C.; they are soluble in common organic solvents and water-insoluble.
[0136] .sub.max lum. in toluene is 570 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com