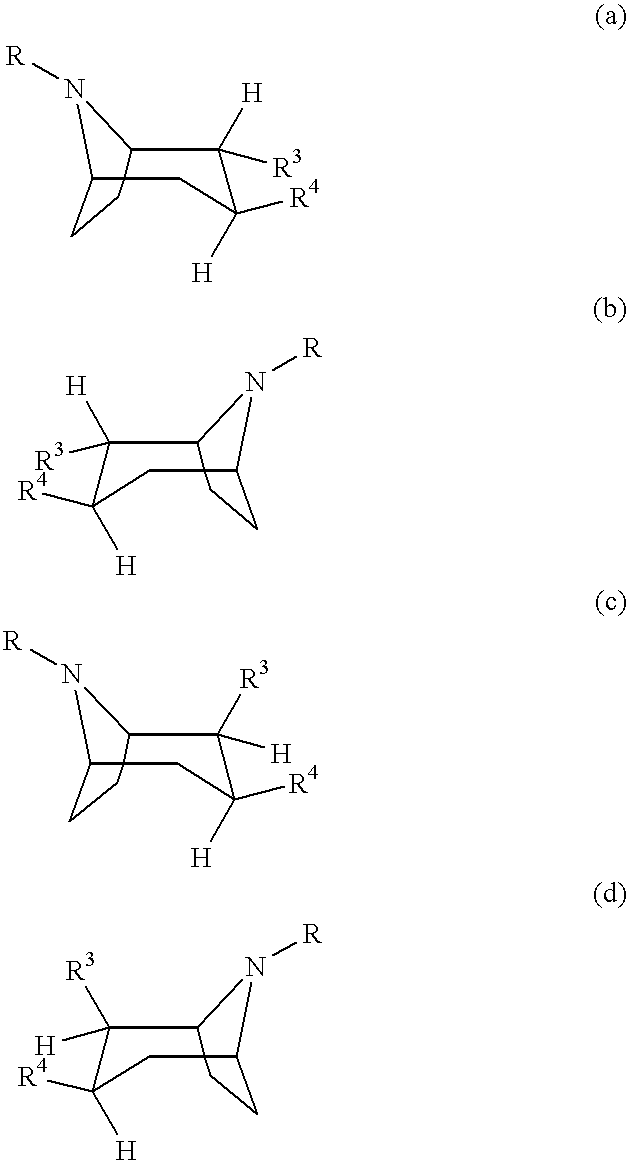
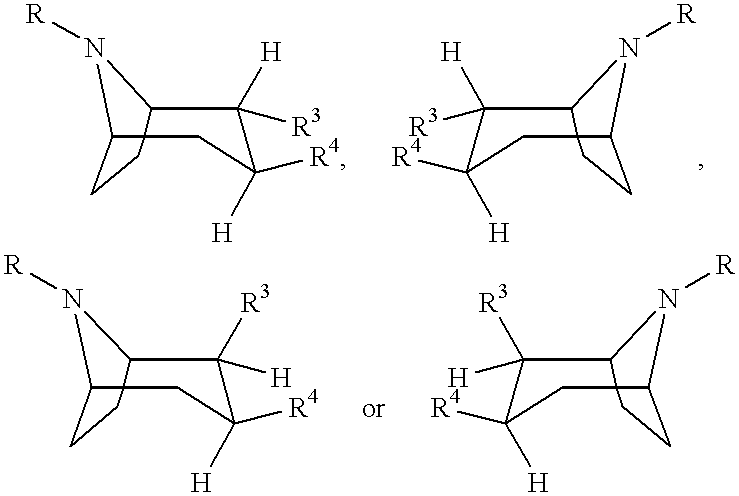
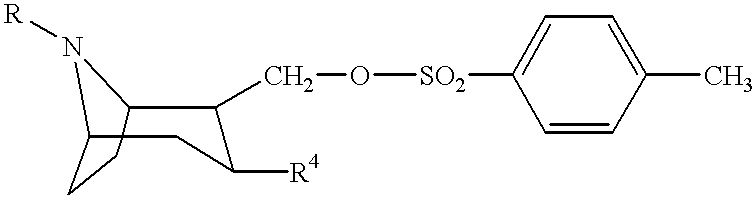
Tropane-derivatives, their preparation and use
a technology of tropane derivatives and derivatives, applied in the field of new drugs, can solve the problems delay in onset of anti-depressant effect, and low efficacy, and achieve the effect of reducing the risk of undesirable central stimulating effect and reducing the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0145] (1R,2S,3S)-2-Carbomethoxy-3-(4-fluorophenyl)tropane and (1R, 2R, 3S)-2-carbomethoxy-3-(4-fluorophenyl)tropane 8
[0146] Grignard reagent was made in a three necked reaction flask equipped with mechanical stirring, an intensive condenser and a pressure equilibrated funnel, using 4bromo-fluorobenzene (27.5 ml, 250 mmol) and magnesium turnings (6.3 g, 260 mmol) in 250 ml absolute diethyl ether. The solution of grignard reagent was cooled to -20.degree. C. and a solution of (-)-anhydroecgonine methyl ester (21.7 g, 120 mmol) in 100 ml absolute diethyl ether was added over 112 hour. The reaction was stirred one hour at -20.degree. C. and the reaction was quenched in one of the following two ways:
[0147] 1) The reaction mixture was stirred into 250 ml crushed ice and the water phase was made acidic by addition of approximately 100 ml 4M hydrochloric acid. The organic phase was discharged and the water phase was washed with 100 ml diethyl ether. The water phase was made basic by additi...
example 3
[0160] (1R, 2R, 3S)-2-Carbomethoxy-3benzyltropane, hydrochloride. 9
[0161] To a solution of (1R, 2S, 3S)-2-carbomethoxy-3-benzyltropane (5.6 g, 20.5 mmol) in absolute methanol (100 ml) was added a solution of sodium methanolate in methanol (2M, 2 ml) and the mixture was refluxed for 16 hours. The reaction mixture was concentrated in vacuo and the residue was dissolved in diethyl ether and was washed with water. The organic phase was dried and concentrated in vacuo. The crude product was purified by column chromatography using a mixture of diethyl ether and pentane (1+1)+1% triethyl amine as eluent yielding (1R, 2R, 3S)-2-carbomethoxy-3-benzyltropane as oil. By dissolution of this product in diethyl ether and subsequent addition of a solution of hydrochloric acid in diethyl ether the title compound precipitated as white crystals, m.p. 188-190.degree. C.
example 4
[0162] 2-Carbomethoxy-3-tropanone. 10
[0163] To a suspension of sodium hydride (3.2 g 80%, 107 mmol, prewashed in cyclohexane) and dimethylcarbonate (9.13 ml, 108 mmol) in absolute cyclohexane heated to reflux temperature, a solution of (+-)-3-tropanone (6.9 g, 50 mmol) in 50 ml absolute cyclohexane was added over 15 minutes. No hydrogen evolution was apparent so 0.2 ml methanol was added. The reaction mixture was stirred over night at reflux temperature and after cooling to ambient temperature 75 ml water was carefully added. To the water phase was added 40 g ammonium chloride and the resulting mixture was extracted 8 times with methylene chloride. The combined methylene chloride organic phases were dried and concentrated in vacuo followed by column chromatography of the crude product using methylene chloride with increasing amounts (up to 10%) of methanol as eluent. The fractions containing the product were concentrated in vacuo and the resulting oil was subjected to Kugelrohr dest...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com