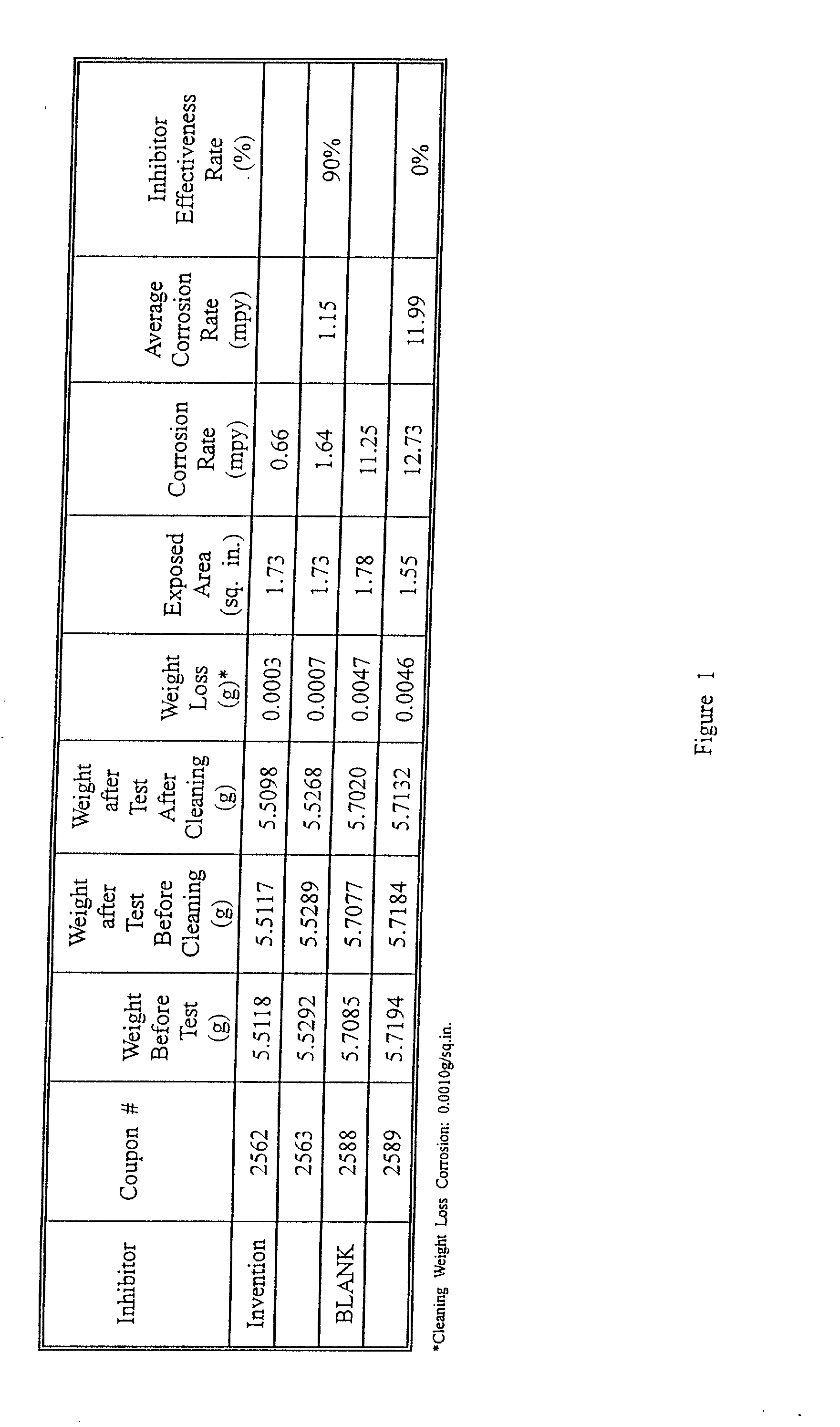
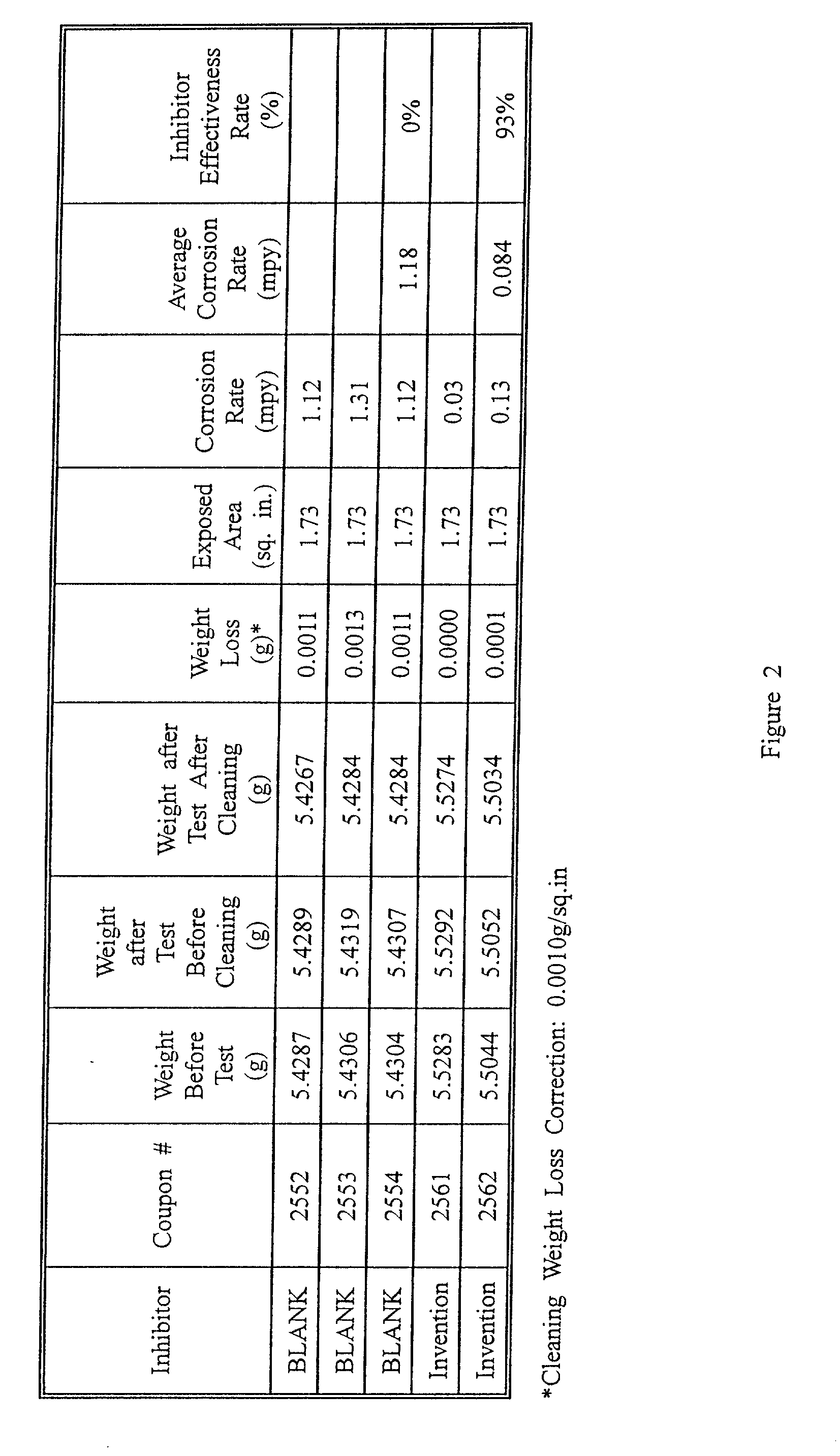
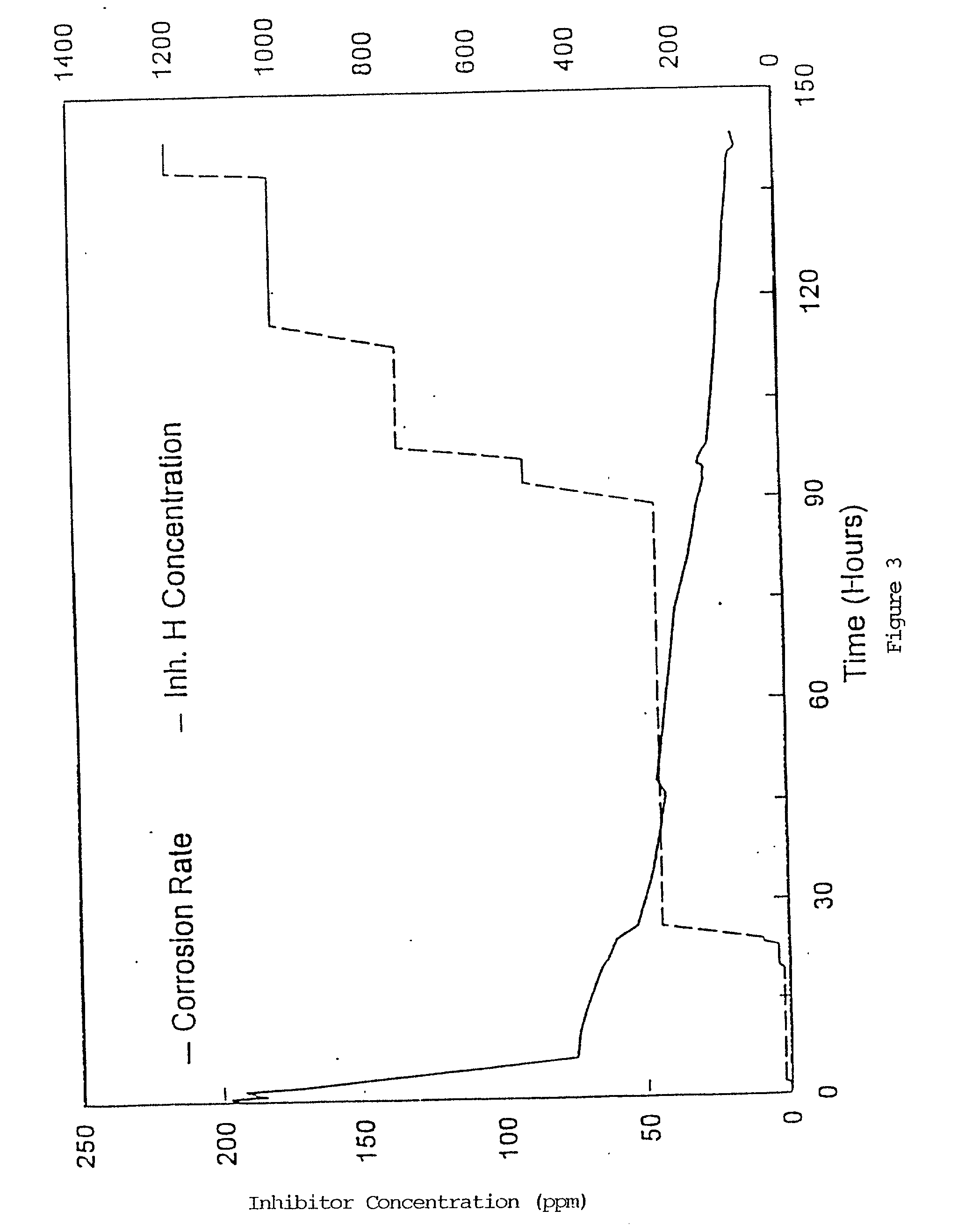
Corrosion inhibitor
a corrosion inhibitor and corrosion technology, applied in the direction of separation process, dispersed particle separation, chemistry apparatus and processes, etc., can solve the problems of general surface corrosion of metals by chemicals dissolved in electrolyte, corrosion problem, and detract from their universal us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0036] An inhibitor composition of the present invention is produced in two stages. In the first stage, about 40 weight percent of a mixture of 85 weight percent monoethanolamine and 15 weight percent water is added to about 60 weight percent of a mixture of 37 weight percent formaldehyde and 7 weight percent methanol in water. In the second stage, the first stage reaction product is titrated with amine heads obtained from Monsanto Chemical to a pH ranging between about 10.5 and about 12.0, or until polymerization occurs and formaldehyde and formaldehyde donor disappears. This occurs when about 14 weight percent amine heads is added to about 86 weight percent of the first stage reaction product. A black liquid product is produced that contains some insoluble polymer, which may precipitate.
example 3
[0037] The product produced by the two-stage reaction of Example 2 is diluted by further mixing with about 5 weight percent of a surfactant, Texaco Surfonic N-150, and an amount of methanol approximately equal in weight to the weight of the product of Example 2.
example 4
[0038] The product produced by the two-stage reaction of Example 2 is diluted by further mixing with about 5 weight percent of a surfactant, cocoamidopropyldimethylbenzyl quaternary ammonium chloride, and an amount of water approximately equal in weight to the weight of the product of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com