Methods of controlling proliferation and differentiation of stem and progenitor cells
a progenitor cell and stem cell technology, applied in the direction of plant growth regulators, biochemical apparatus and processes, biocide, etc., can solve the problems of halting cell division, limiting the differentiation process, severe cases of shwanchman syndrome, etc., to increase ra-induced differentiation, increase copper content per protein content, and increase the effect of copper uptak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
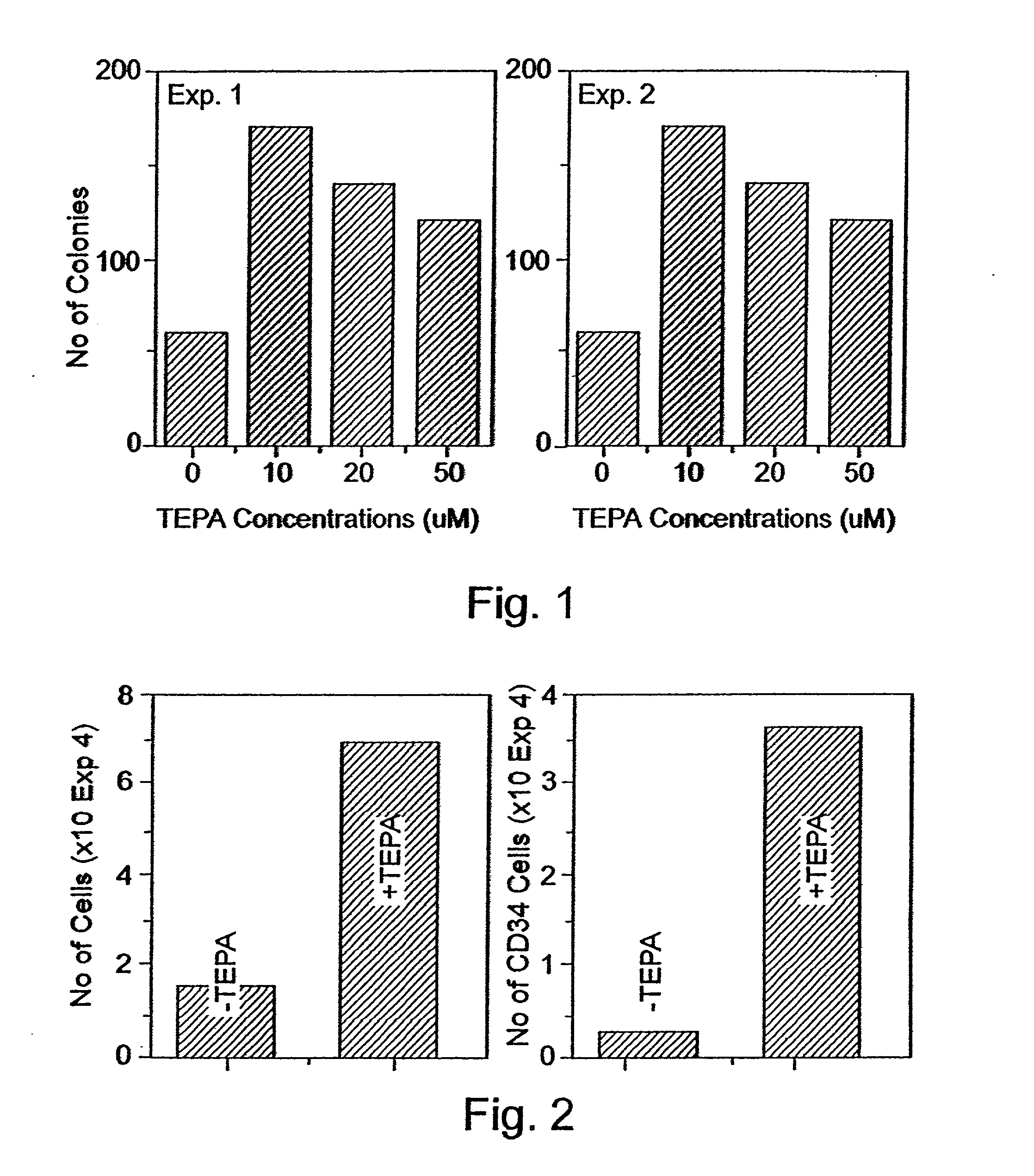
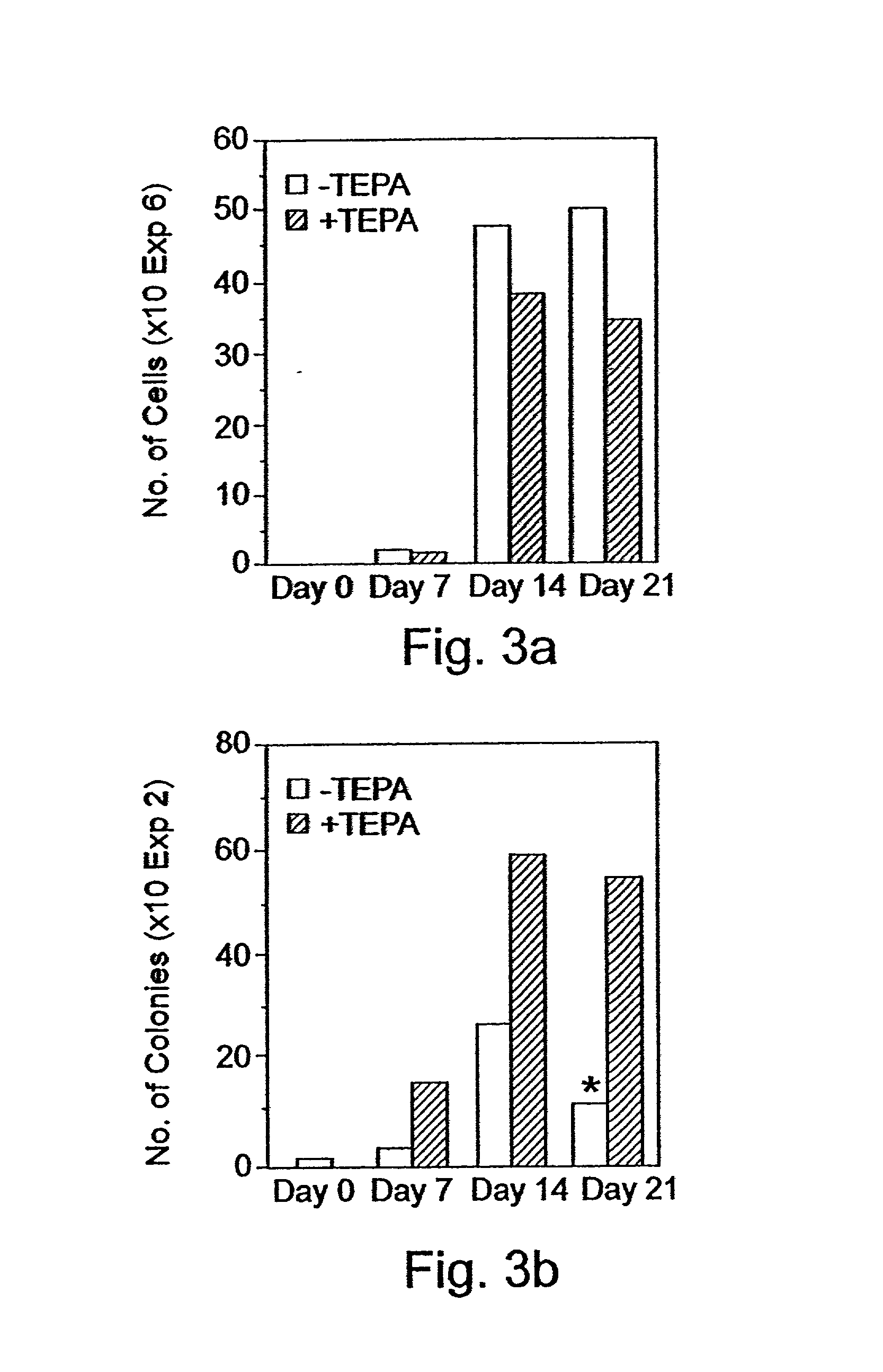
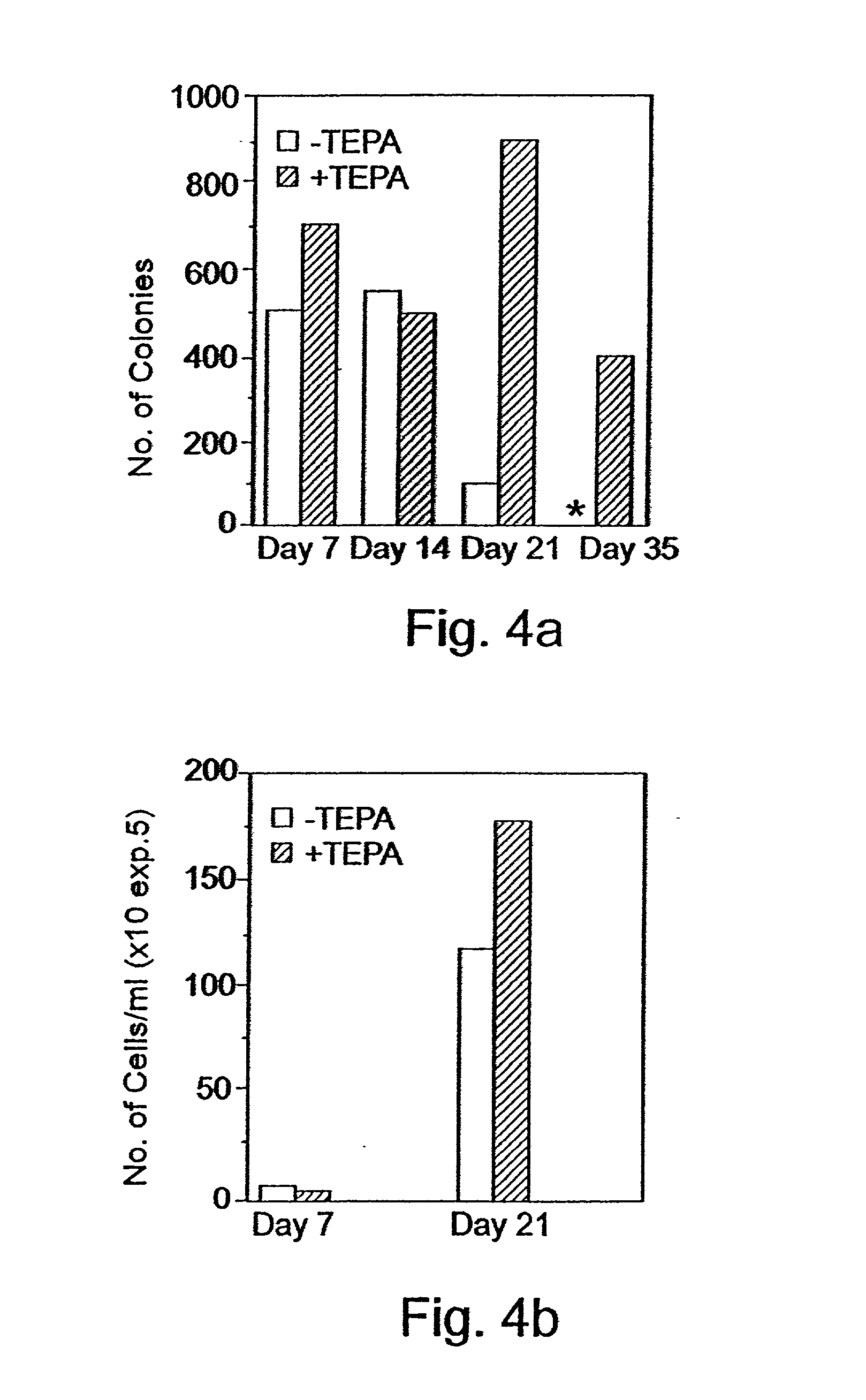
Imposing Proliferation yet Restricting Differentiation of Stem and Progenitor Cells by Treating the Cells with Chelators of Transitional Metals
Experimental Procedures
[0241] CD.sub.34 Cells Selection:
[0242] Peripheral blood "buffy coat" cells derived from a whole blood unit, peripheral blood cells obtained following leukapheresis, or cord blood cells were layered on Ficoll-Hypaque (density 1.077 g / ml) and centrifuged at 1,000.times. g for 20 min. at room temperature. The interphase layer of mononuclear cells were collected, washed three times with Ca / Mg free phosphate buffered saline containing 1% bovine serum albumin (BSA). The cells were incubated for 30 mm. at 4 .degree. C. with murine monoclonal anti CD.sub.34 antibody (0.5 .mu.g / 10.sup.6 mononuclear cells) and thereafter isolated using the miniMACS apparatus (Miltenyi-Biotec, Bergisch, Gladbach, Germany) according to the manufacturer's protocol.
[0243] Culture Procedures:
[0244] For the expansion of progenitor cells, CD.sub.34.sup...
example 2
The effect of Copper-Chelating Peptides on Proliferation and Clonability in CD.sub.34 Cell Cultures
Experimental Procedures
[0305] CD.sub.34 Cells Selection:
[0306] Peripheral blood "buffy coat" cells derived from a whole blood unit, peripheral blood cells obtained following leukapheresis, or blood cells were layered on Ficoll-Hypaque (density 1.077 g / ml) and centrifuged at 1,000.times. g for 20 minutes at room temperature. The interphase layer of mononuclear cells were collected, washed three times with Ca / Mg free phosphate buffered saline containing 1% bovine serum albumin (BSA). The cells were incubated for 30 minutes at 4.degree. C. with murine monoclonal anti CD.sub.34 antibody (0.5 .mu.g / 10.sup.6 monoclonal cells) and thereafter isolated using the miniMACA apparatus (Miltenyl-Biotec, Bergisch, Gladbach, Germany) according to the manufacturers protocol.
[0307] Culture Procedures:
[0308] For the expansion of progenitor cells, CD.sub.34.sup.+ enriched fractions were seeded at 1.times....
example 3
Transition Metal Chelator Assay for Determining the Effect of a Specific Transition Metals Chelator on Cell Differentiation
Experimental Procedures
[0319] Inhibition of Differentiation:
[0320] MEL (mouse erythroleukemia cell line), 8.times.10.sup.3 cells per ml were incubated for 24 hours with different chelators at concentrations indicated in Table 3 below. Then, cultures were supplemented with a differentiation inducer--hexamethylene bisacetamide, 2 mM. Number of cells and percentage of differentiated cells (benzidine positive) were determined 72 hours after addition of the inducer.
[0321] Similarly, HL-60 (human myeloid leukemia cell line), 1.times.10.sup.5 cells per ml were incubated for 24 hours with different chelators at the concentrations indicated in Table 3 below. Then, cultures were supplemented with the differentiation inducers--vitamin D or retinoic acid (both at 1.times.10.sup.-7 M). Number of cells and percentage of differentiated phagocytosing) cells were determined.
[032...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com