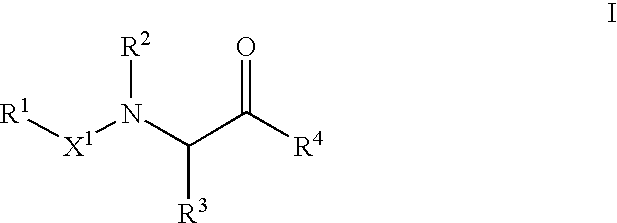
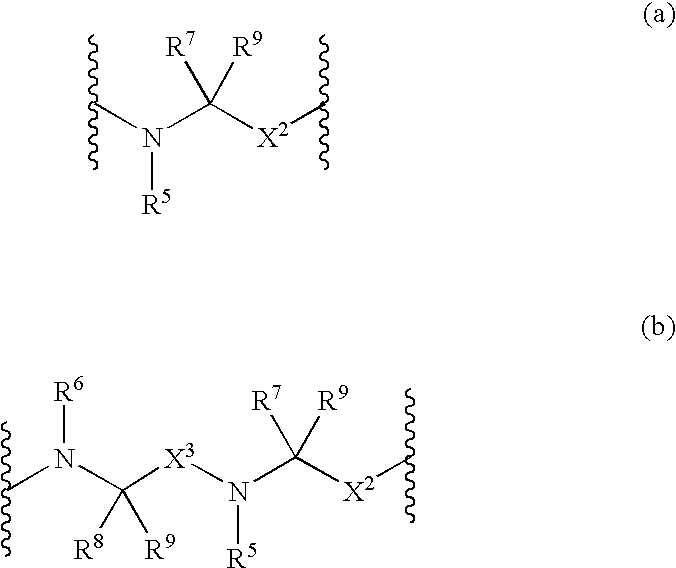
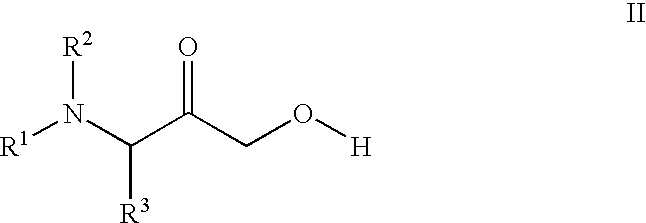
Novel compounds and compositions as protease inhibitors
a technology of protease inhibitors and compounds, applied in the direction of dipeptides, peptide/protein ingredients, depsipeptides, etc., can solve the problems of pathological consequences and the activeness of cysteine proteases, and achieve the effect of preventing, inhibiting or ameliorating the pathology and/or symptomatology of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Benzyl 1S-(3-hydroxy-2-oxo-1S-phenethylpropylcarbamoyl)-3-methylbutylcarba-mate
[0204] 26
[0205] A mixture comprised of magnesium turnings (235 mg, 9.58 mmol) and mercuric chloride (35 mg, 0.13 mmol) in dry THF under nitrogen was cooled to between -10 and -20.degree. C. and chloromethoxymethane (0.75 mL, 9.58 mmol) was added. The mixture was stirred for 6 hours while the temperature was allowed to warm to between -8 and 0.degree. C. The mixture was then cooled to -78.degree. C. and stirred while a solution comprised of benzyl 1-[1-(N-methoxy-N-methylcarbamoyl)-3-phenylpropylcarb-amoyl]-3-methylbutylcarbamate (500 mg, 1.08 mmol) in anhydrous THF (6 mL) was added. The mixture was allowed to warm slowly over approximately 12 hours and then the reaction was quenched with ammonium chloride solution and then extracted with ethyl acetate. The ethyl acetate was dried (MgSO.sub.4), filtered and concentrated. Product was purified from the residue by flash column chromatography eluting with 33:1...
example 2
3S-(2S-Benzyloxycarbonylamino-4-methylpentanoylamino)-2-oxo-5-phenylpentyl 2,5-dichlorobenzoate (Compound 7),
[0212] 27
[0213] A solution comprised of (S)-2-benzyloxycarbonylamino-4-methylpentan-oic acid (0.20 g, 0.76 mmol) in THF (5 mL) was cooled to -10.degree. C. and then 4-methylmorpholine (84 .mu.L, 0.76 mmol) and isobutyl chloroformate (99 .mu.L, 0.76 mmol) were added. The mixture was stirred for 5 minutes and then (S)-3-amino-2-oxo-5-phenylpentyl 2,5-dichlorobenzoate toluenesulfonic acid salt (0.41 g, 0.76 mmol) and 4-methylmorpholine (84 .mu.L, 0.76 mmol) were added sequentially. The mixture was stirred for 45 minutes and then ethyl acetate (30 mL) was added. The mixture was washed with 1M hydrochloric acid (5 mL), saturated aqueous sodium bicarbonate (5 mL) and brine (5 mL), dried (MgSO.sub.4), filtered and concentrated. The residue was crystallized from CH.sub.2Cl.sub.2 / ether to provide 3S-(2S-benzyloxycarbonylamno-4-methylpe-ntanoylamino)-2-oxo-5-phenylpentyl 2,5-dichlorobe...
example 3
Benzyl 1S-(3-hydroxy-2-oxo-1S-phenethylpropylcarbamovl)-3-methylbutylcarba-mate
[0281] 28
[0282] Potassium carbonate (31 mg, 0.225 mmol) was added to a solution comprised of the 3S-- (2S-benzyloxycarbonylamino-4-methylpentanoylamino)--2-oxo-5-phenylpentyl 2,5-dichlorobenzoate (0.92 g, 1.5 mmol), provided as in Example 2, in 1:1 methanol / THF (40 mL). The mixture was stirred for 60 minutes and then 1M hydrochloric acid (2 mL) was added. The mixture was concentrated in vacuo at room temperature and the residue was dissolved in ethyl acetate (40 mL). The solution was washed with 1M hydrochloric acid (5 mL) and saturated aqueous sodium bicarbonate (5 mL), dried (MgSO.sub.4), filtered and concentrated. Product was purified from the residue by flash chromatography (50% CH.sub.2Cl.sub.2 / ethyl acetate) to provide benzyl 1S-(3-hydroxy-2-oxo -1S-phenethylpropylcarbamoyl)-3-methyl-butylcarbamate (0.37 g, 0.84 mmol) in approximately a 3:1 mixture of (S,S)- to (S,R)-diastereomers. .sup.1H NMR (CDCl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com